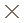송시영 교수님이 발표한 캡슐 내시경 2가지 제품인 미로캠과 필캠의 비교논문입니다.
2가지 캡슐이 이미지 전송 방식 자체가 다르기 때문에 전력 소모량과 배터리 구동시간이 다릅니다.
백그라운드/목표
새롭고 혁신적인 데이터 전송 방식을 이용한 미로캠 (아마도 E filed propagation 지칭하는 듯)과 과거의 필캠을 비교한 논문은 없었습니다.
방법
12시간 금식후 랜덤방식으로 첫 번째 캡슐을 삼키고 위장을 넘어가는 것을 방사선으로 확인한 뒤 두번째 캡슐을 복용함.
결과
24명의 환자분을 대상으로 맹장까지 도달율은 미로캠은 83.3% 였고 필캠은 58.3%였다.
진단적 검출률은 미로캠은 45.8%, 필캠은 41.7%였다. 진단 일치율은 87.5%였다. 7명의 환자에서 전송방식간 간섭이 관찰되었다.
결론
미로캠과 필캠의 캡슐내시경 검사는 통계적으로 유의하지는 않지만 진단률에 약간의 차이가 있었고 두가지 종류의 캡슐에 전기적 간섭은 없었다. 연속적인 캡슐내시경에 대한 추가적인 유용성에 대한 연구가 필요하다.
Gut Liver. 2010 Jun;4(2):192-200.
Published online 2010 June 16. http://dx.doi.org/10.5009/gnl.2010.4.2.192
A Pilot Study of Sequential Capsule Endoscopy Using MiroCam and PillCam SB Devices with Different Transmission Technologies
Si Young Song*†
*Division of Gastroenterology, Department of Internal Medicine and Yonsei Institute of Gastroenterology, Yonsei University College of Medicine, Seoul, Korea.
†Brain Korea 21 Project for Medical Science, Yonsei University College of Medicine, Seoul, Korea.
Correspondence to: Si Young Song. Division of Gastroenterology, Department of Internal Medicine, Yonsei University College of Medicine, 250, Seongsanno, Seodaemun-gu, Seoul 120-752, Korea. Tel: +82-2-2228-1957, Fax: +82-2-2227-7900, Email: sysong@yuhs.ac
Received September 18, 2009; Accepted December 29, 2009.
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article has been cited by 13 articles in
This article has been cited by 1 article in KoMCI.
This article has been cited by Google Scholar.
Abstract
Background/Aims
Studies have investigated the use of different types of radiofrequency capsules for comparison or sequential capsule endoscopy, but none have compared the MiroCam device – which utilizes a novel data transmission technology – with other capsules. This study compared the feasibility of sequential capsule endoscopy using the MiroCam and PillCam SB devices, which employ different transmission technologies.
Methods
Patients with diseases requiring capsule endoscopy were enrolled. After a 12-hour fast, one randomly selected capsule was swallowed. The second capsule was swallowed once fluoroscopy had indicated that the first capsule had migrated below the gastric outlet.
Results
The total operating time in 24 patients was 702±60 min (mean±SD) for the MiroCam and 446±28 min for the PillCam SB (p<0.0001). The rate of a complete examination to the cecum was 83.3% for the MiroCam and 58.3% for the PillCam SB (p=0.031). Diagnostic yields for the MiroCam, PillCam SB, and sequential capsule endoscopy were 45.8%, 41.7%, and 50.0%, respectively. The agreement rate between the two capsules was 87.5%, with a κ value of 0.74. Electrical interference in data transmission between the two capsules was not observed, but temporary visual interferences were observed in seven patients (29.2%).
Conclusions
Sequential capsule endoscopy with the MiroCam and PillCam SB produced slight but nonsignificant increases in the diagnostic yield, and the two capsules did not exhibit electrical interference. A larger trial is necessary for elucidating the usefulness of sequential capsule endoscopy.
Keywords: Capsule endoscopy, Gastrointestinal hemorrhage, Feasibility study, Diagnosis.
INTRODUCTION
M2A (renamed PillCam SB; Given Imaging, Yokneam, Israel) was the first developed wireless capsule endoscope providing noninvasive and visual diagnosis of small bowel disease. Capsule endoscopy (CE) provides great diagnostic value in various small bowel diseases compared to conventional modalities.1,2 Recently, other new wireless capsule endoscopes including EndoCapsule (Olympus, Tokyo, Japan) and MiroCam (IntroMedic, Seoul, Korea), have become available for small bowel examination in clinical situations.3,4 Among them, MiroCam received both Korean market clearance and the Conformité Européenne (CE) mark in 2007.
MiroCam uses a novel technology for data transmission, Human Body Communication, which is different from the radiofrequency telemetry of PillCam SB and EndoCapsule.3 Human Body Communication uses the human body as a conductive medium for data transmission from the capsule endoscope to electrodes attached to the body.4 As a result, this technology spends less electrical power for data transmission, confers a longer operating time with a small size (24×11 mm), and provides higher-resolution images (320×320 pixels at 3 frames per second) compared to PillCam SB.4 Longer operating times may reduce the rate of incomplete examinations to the cecum by CE, which has been reported to be 17-25% throughout the lifetime of the battery.5,6 However, no prospective randomized trial has been reported in diseased patients to compare the operating times, complete examination rates to the cecum, and diagnostic yields between MiroCam and PillCam SB.
The concept of sequential CE originated from previous studies on repeat examinations with CE. Repeat CE and second-look CE in selected patients with non-diagnostic results during their first CE have identified additional new findings in patients with recurrent or persistent obscure gastrointestinal (GI) bleeding and iron deficiency anemia.7-9 Therefore, sequential CE with two capsules in all subjects could increase diagnostic yield, compared to a single CE.
We tested the feasibility of sequential CE utilizing MiroCam and PillCam SB, and also compared two capsule endoscopes in a prospective randomized design.
MATERIALS AND METHODS
1. Patients
Patients with an indication for CE, such as obscure GI bleeding, chronic abdominal pain, and chronic diarrhea were enrolled. Patient ages ranged from 20 to 75 years. Patients with a history of intestinal obstruction and those with swallowing difficulties were excluded. The patients with obscure GI bleeding underwent esophagogastroduodenoscopy and colonoscopy prior to CE examination. Approval was obtained from the institutional review board of Severance Hospital (Seoul, Korea). Written informed consent was obtained from each patient.
2. Procedures of sequential CE
Enrolled patients fasted for 12 hours, and on the day of examination, swallowed 30 mL of simethicone 30 minutes before the first CE. The order of swallowing two capsules was randomized. Eight antenna or electrodes were attached to the abdominal wall according to the manufacturer’s instructions. The first capsule endoscope was swallowed with water, and its location was traced under fluoroscopy at 30 minutes intervals. After the first capsule endoscope passed through the gastric outlet, the second capsule endoscope was ingested with water. Both the antennas and electrodes were detached simultaneously after sufficient battery time of MiroCam or PillCam SB. Data from each capsule endoscope were uploaded to respective computer installed with the appropriate reading software.
3. Data analysis
Two gastroenterologists interpreted the images from each capsule endoscope, and were kept from knowing the results from the other capsule endoscope. When two gastroenterologists reviewing the same patient were not in agreement, a third gastroenterologist assisted in the interpretation of the images. The readers were not completely blinded because the reading programs were clearly distinct. The personal history and information from other modalities were not informed to the readers. Abnormal findings of CE images were labeled according to the Capsule Endoscopy Structured Terminology (CEST).10 We classified mucosal lesions of the small bowel into three categories. Significant abnormal findings were defined as mucosal lesions strongly associated with disease. Abnormal findings were defined as any mucosal lesion. Clinical diagnoses were made based on clinical signs and symptoms of the patients and interpretation of images with significant abnormal findings. Diagnostic yield was calculated as the number of patients with significant abnormal findings divided by the number of all patients.
Operating times of the two capsule endoscopes were compared with the paired t-test, and completion rates and diagnostic yields were compared using McNemar test. The kappa test was used to measure an agreement between MiroCam and PillCam SB. p-values were considered to be significant when they were less than 0.05.
RESULTS
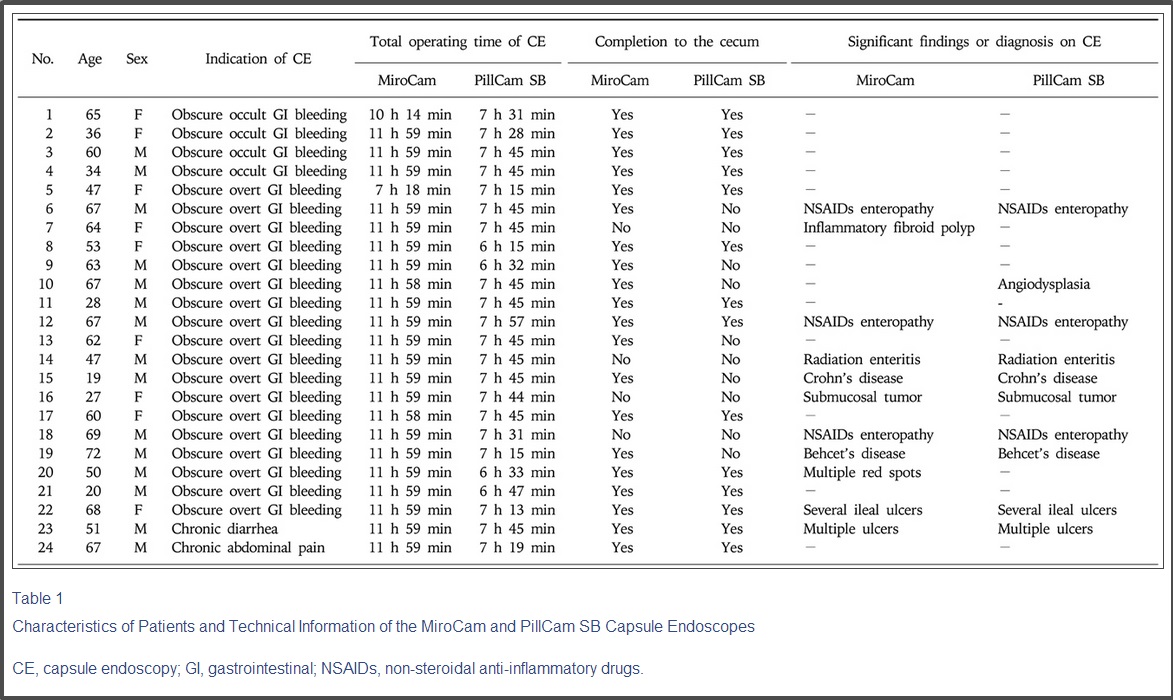
Twenty four patients were enrolled between June 2007 and January 2009 (Table 1).
24명의 환자분의 성별, 나이, 구동시간과 밝혀낸 원인 질환에 대한 내용입니다.
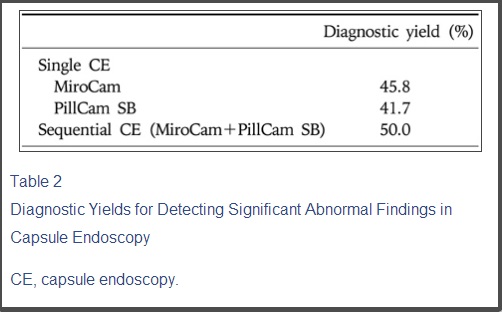
The mean age was 52.6±16.7 years. Indications of CE were obscure GI bleeding in 22 patients (91.7%) with a lowest hemoglobin concentration of mean 8.2±2.0 g/dL, chronic diarrhea in one patient, and abdominal pain in one patient. The mean interval time of ingestion between MiroCam and PillCam SB was 3 h 7 min±3 h 58 min. The mean total operating times of MiroCam and PillCam SB were 11 h 44 min±1 h, and 7 h 26 min±28 min (p<0.0001), respectively. Completion rates to the cecum by MiroCam and PillCam SB was 83.3% and 58.3%, respectively (p=0.031). Diagnostic yields of MiroCam and PillCam SB were 45.8% and 41.7% (p>0.05), respectively. The sequential CE of two capsules slightly increased the diagnostic yield to 50.0% compared to a single CE, but this difference was not statistically significant (Table 2).
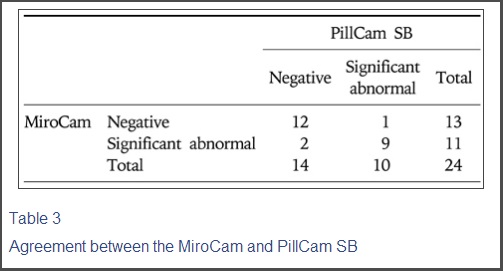
미로캠과 필캠의 진단 일치율에 대한 자료
The agreement rate between MiroCam and PillCam SB was 87.5% with a kappa value of 0.74 (Table 3).
미로캠과 필캠의 의미있는 소견에 대한 진단율 차이 비교
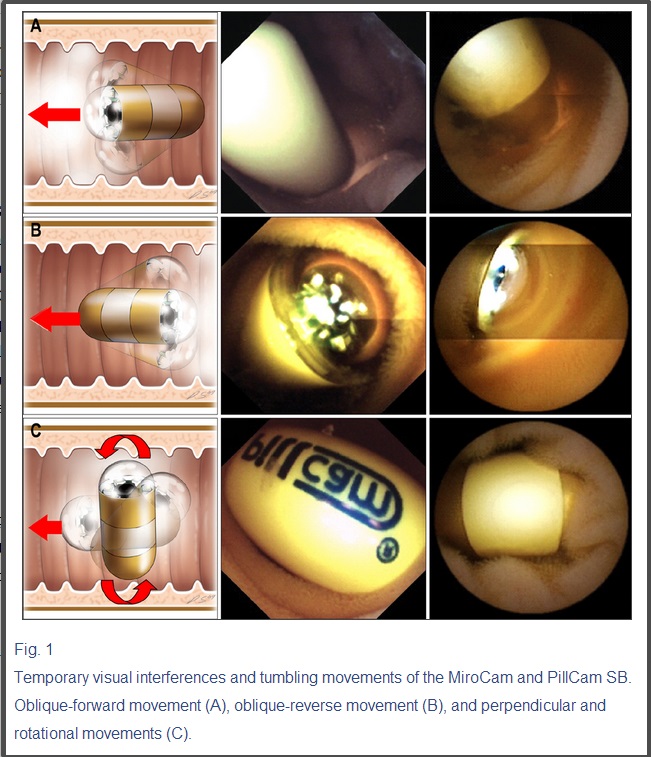
No complication related to CE occurred. Interference of electrical transmission between MiroCam and PillCam SB was not observed in any patient, and images were captured and transferred completely during the lifetime of the battery. However, temporary visual interferences disturbing front visual fields due to physical obstruction of the capsule were observed in seven patients (29.2%) (Fig. 1).
Figure F1
일시적인 전기적 간섭현상과 캡슐내시경의 소장내에서 텀블링 움직임 모습.
캡슐 내시경은 검사중 순방향으로 십이지장을 진입해 들어갈 수도 있고, 역방향으로 들어갈 수도 있습니다. 또한 좁은 소장내에서 연동운동에 따라 앞뒤로 제자리에서 움직이는 경우도 있고 뱅글뱅글 돌아가는 경우도 있습니다.
소장 점막 주름의 병변 위치에 따라 진단율이 차이가 날 수 있습니다.
Fig. 1
Temporary visual interferences and tumbling movements of the MiroCam and PillCam SB. Oblique-forward movement (A), oblique-reverse movement (B), and perpendicular and rotational movements (C).
DISCUSSION
The concept of second-look CE was first reported in 2004.8 “Repeat CE” have revealed new findings not previously observed in prior CE in patients with obscure GI bleeding in two studies.7,9 The second-look CE or repeat CE was performed on selected patients with negative finding in prior CE, but the sequential CE was a procedure of continuous swallowing two capsules in all patients. The sequential CE has been prospectively attempted in three studies.11-13 One of them, using two same capsules of PillCam SB, reported that the second capsule found 10% more new lesions in 41 patients with obscure GI bleeding.13 In other trial, the combination of PillCam SB and EndoCapsule showed increase in the diagnostic yield from 33.3% or 47.1% of each single capsule endoscope up to 52.9% in patients with obscure GI bleeding.12 In another study, the combination of PillCam SB (65%) and EndoCapsule (72.5%) revealed 77.5% of the detection rate of abnormal lesions in 40 patients with obscure GI bleeding.11 Our study showed that the combination of MiroCam and PillCam SB had 50% of the detection rate in 22 patients with obscure GI bleeding. However, we did not prove the significant efficacy of sequential CE, compared to single CE. Further trials for investigation of the efficacy of sequential CE will be needed.
Since the development of M2A, several different types of capsule endoscopes have become commercially available. EndoCapsule was developed by Olympus Medical System, and utilizes charged coupled device (CCD) technology for the image sensor, rather than the complementary metal oxide semiconductor (CMOS) of PillCam SB and MiroCam.3 EndoCapsule had a comparable diagnostic yield to PillCam SB for obscure GI bleeding in two randomized trials.11,12 In addition, EndoCapsule had a significantly longer recording time than PillCam SB (591 min vs 471 min, respectively), and a higher frequency of identifying abnormal findings than PillCam SB (47.1% vs 33.3%).11,12 MiroCam uses a different technology for data transmission from PillCam SB and EndoCapsule. Our study was the first pilot study to directly compare MiroCam and PillCam SB, and demonstrated that MiroCam had longer operating time than PillCam SB, and a comparable diagnostic yield to PillCam SB. Our study showed the relatively low diagnostic yields for obscure GI bleeding (45.5% in MiroCam and 40.9% in PillCam SB among 22 patients), but the low values are close to rates of other studies.1,5
EndoCapsule does not electrically interfere with PillCam SB, even when operating in the same patient, although both the capsules use the same radiofrequency communication for data transmission.11,12 MiroCam also did not interact with PillCam SB in our study. Two of the same capsules will cause electrical interference when operating simultaneously in the same patient on the same day. Therefore, a long lag-time greater than the battery lifetime of the capsule is needed to successfully perform sequential CE with two same capsules.13 Sequential CE of two different capsules can be performed in the same day, after a short time interval to avoid visual interference. Moreover, sequential CE may increase the detection rate of abnormal findings in the small intestine.
In our study, MiroCam had a higher complete examination rate to the cecum than PillCam SB. The completion rate of PillCam SB was lower than those in other reports.6 However Rastogi et al.14 reported 50% of incompletion rate of PillCam SB. This low rate might be caused by sampling bias. If an abnormal lesion is located in the distal ileum, MiroCam is more likely to detect it than PillCam SB. For example, in one patient, MiroCam found a large polypoid mass at 11 h 31 min in the distal ileum, whereas PillCam SB was unable to detect the lesion because its short battery lifetime expired after 7 h 45 min (Fig. 2). The risk factors for incomplete examination of CE in the small bowel are long gastric transit time, previous small bowel surgery, hospitalization, and poor bowel cleansing in a retrospective study using PillCam SB.15,16 Sufficiently long operating times of capsule endoscope can overcome some portion of these risk factors.
실제 인트로메딕사와 과거 전화 통화시 11시간 59분 이상의 구동시간을 작동시킬 수 있지만 사진의 갯수와 판독의 용이성이나 여러가지 이유로 일부러 12시간 이내에 검사를 종료하게 셋팅이 되어 있다고 들었습니다.
Figure F2
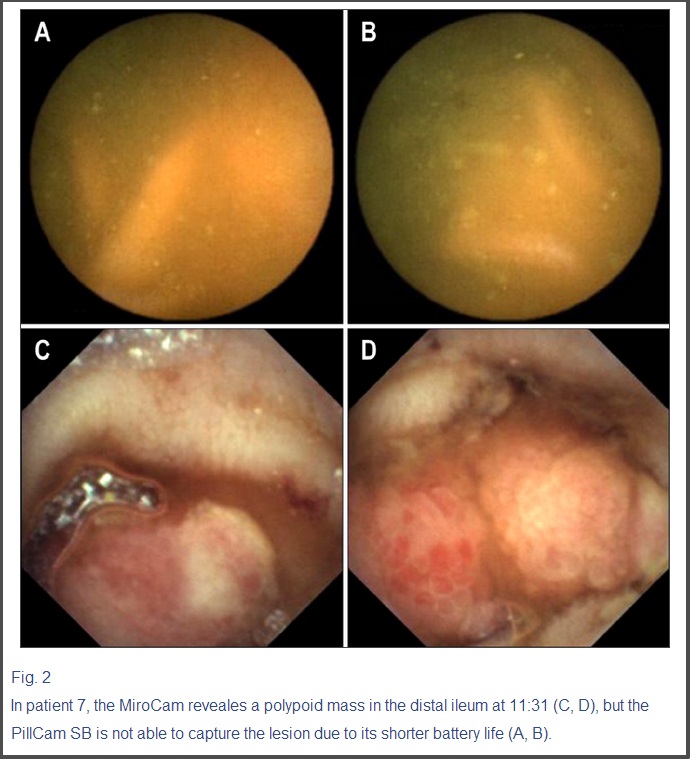
In patient 7, the MiroCam reveales a polypoid mass in the distal ileum at 11:31 (C, D), but the PillCam SB is not able to capture the lesion due to its shorter battery life (A, B).
짧은 배터리 구동시간은 말단 회장부위의 중요한 병변을 놓칠 수 있습니다.
Indications of CE include obscure GI bleeding, small bowel Crohn’s disease, small bowel tumors, celiac disease and abdominal pain. In our study, obscure GI bleeding was a major indication (91.7%) of CE although all indications of CE were included. High prevalence of obscure GI bleeding might cause this selection bias. In addition, other indications, small bowel Crohn’s disease, small bowel tumors, celiac disease and abdominal pain, have low diagnostic rate of positive findings on CE, and therefore a lot of subjects may be necessary for evaluation of efficacy of sequential CE.17-19
It is well documented that nonsteroidal anti-inflammatory drug (NSAID) can cause small bowel mucosal injury, such as red spot, erosion, ulcer and stricture, since CE has become available for evaluation of small bowel.20 This NSAID-induced enteropathy is often found as an origin of obscure GI bleeding in patient taking NSAID. Recently, aspirin is also suggested to cause enteropathy similar to NSAID-induced enteropathy although its association of cause and effect remains clarified.21 In our study, three of six patients taking aspirin were diagnosed with NSAID-induced enteropathy. One patient taking aceclofenac had negative finding in small bowel on CE.
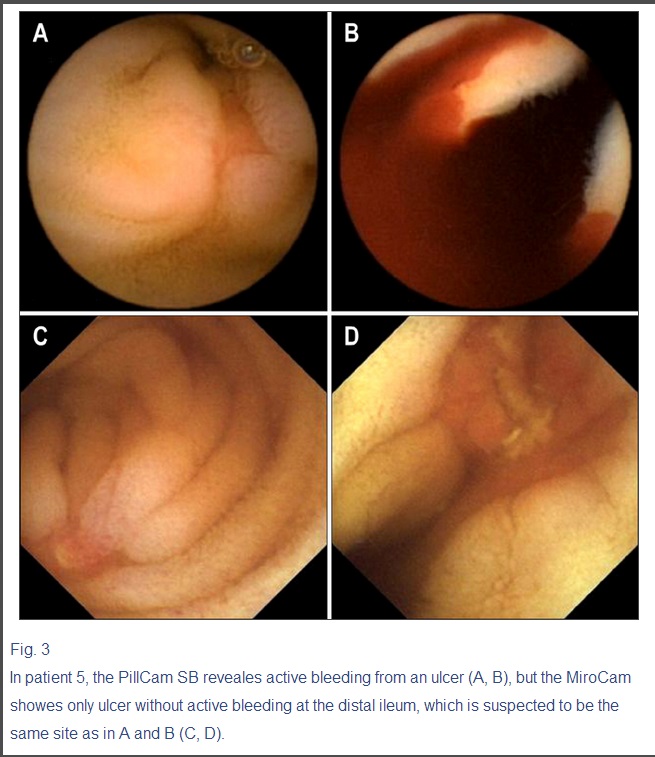
The swallowing order of two capsules did not cause any significant difference in operating time and diagnostic yield (The data were not shown in the Result Section). However, one capsule could detect active bleeding focus, while the other capsule could detect an ulcer without current bleeding at the same site. For example, PillCam SB was the second capsule in one patient (Fig. 3), and it detected active bleeding in the small intestine; however, MiroCam, the first capsule swallowed 1 h 50 min before, found an ulceration without current bleeding at the same site.
궤양부위의 출혈 소견에 대한 detection rate는 검사 장비의 차이라기 보다는 소장 연동운동과 캡슐 방향에 따른 랜덤한 결과로 여겨집니다.
Fig. 3
In patient 5, the PillCam SB reveales active bleeding from an ulcer (A, B), but the MiroCam showes only ulcer without active bleeding at the distal ileum, which is suspected to be the same site as in A and B (C, D).
In our study, two capsules were ingested separately to avoid visual interference, but in seven patients, the two capsule endoscopes were intermittently close to each other in the small bowel to partially interrupt the visual field of one or both by physical obstruction, or by light when the light-emitting diode (LED) was on, as shown Fig. 1. Two capsule endoscopes can interfere with each other visually if the times of ingestion are not adequately separated. Cave et al.12 reported intermittent visualization of the other capsule endoscope with a 40-minute interval between ingestion. To prevent the encounter of two operating capsule endoscopes, the time interval for ingestion should be at least 1 hour.
The tumbling movement of capsule endoscopes was first observed in a comparison study of EndoCapsule and PillCam SB.12 The present study showed that the tumbling movements included oblique inclination and rotation, and that the capsule endoscope rapidly changed its position in the small intestine. The tumbling movements are illustrated in Fig. 1. When the capsule endoscope is positioned randomly, it causes a blind area in the small bowel mucosa that decreases the diagnostic yield.12
텀블링 무브번트로 인한 맹점 부위의 가능성 설명
Our study has two limitations. Number of the enrolled patients were small although of a pilot study. A head-to-head prospective study between MiroCam and PillCam SB has not been previously evaluated, and therefore we tried to compare two capsule endoscopes. The results of our study showed that MiroCam was comparable to PillCam SB in diagnostic yield; however larger scale trials are necessary to compare two capsule endoscopes. The other problem is the failure to blind the reviewer of CE images. The reviewers were gastroenterologists and employed in the same hospital where our study was performed, but they were prohibited from participation in care of the enrolled patients and were not given information about the patients. However, the complete blindness could not be achieved because the reading programs of two capsule endoscopes were obviously different, and the patients’ names were showed on the reading programs. In addition, evaluation of image quality was not performed in our study (Figs 4 and 5).
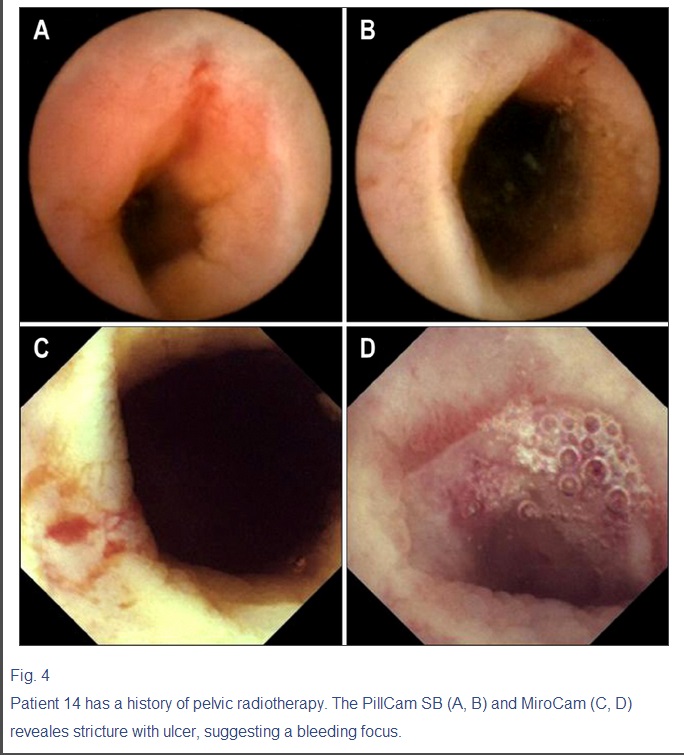
Fig. 4
Patient 14 has a history of pelvic radiotherapy. The PillCam SB (A, B) and MiroCam (C, D) reveales stricture with ulcer, suggesting a bleeding focus.
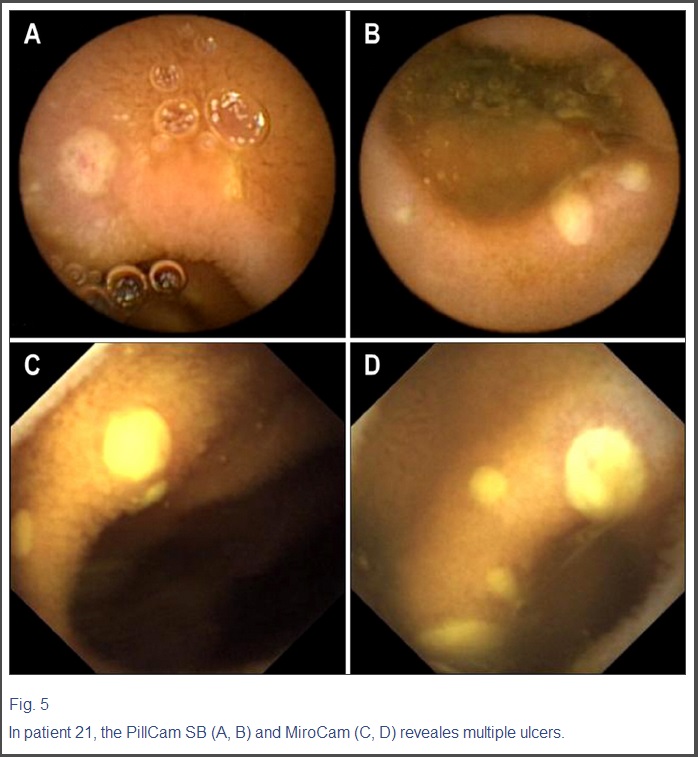
Fig. 5
In patient 21, the PillCam SB (A, B) and MiroCam (C, D) reveales multiple ulcers.
In conclusion, sequential CE with MiroCam and PillCam SB showed slight increase in the diagnostic yield, but it was not statistically significant. Two capsule endoscopes did not cause interference from each other. Further trails to test the efficacy of sequential CE using two different capsule endoscopes will be necessary. As subsidiary findings, MiroCam had longer operating time, higher completion rate, and comparable diagnostic yield, compared to PillCam SB in the small bowel disease.
ACKNOWLEDGEMENTS
This study was supported in part by a grant from the 21st Century Frontier R&D Project sponsored by the Korean Ministry of Knowledge Economy, by a grant from the Brain Korea 21 Project for Medical Sciences of Yonsei University College of Medicine, and by IntroMedic Co., Ltd., Seoul, Korea. Special thanks go to Jin Suk Jang of Yonsei Clinical Trial Center, Seoul, Korea, for helpful cooperation and Dong-Su Jang for the illustrations.
References
Triester SL, Leighton JA, Leontiadis GI, et al. A meta-analysis of the yield of capsule endoscopy compared to other diagnostic modalities in patients with obscure gastrointestinal bleeding. Am J Gastroenterol 2005;100:2407–2418.
Marmo R, Rotondano G, Piscopo R, Bianco MA, Cipolletta L. Meta-analysis: capsule enteroscopy vs. conventional modalities in diagnosis of small bowel diseases. Aliment Pharmacol Ther 2005;22:595–604.
Gheorghe C, Iacob R, Bancila I. Olympus capsule endoscopy for small bowel examination. J Gastrointestin Liver Dis 2007;16:309–313.
Bang S, Park JY, Jeong S, et al. First clinical trial of the “MiRo” capsule endoscope by using a novel transmission technology: electric-field propagation. Gastrointest Endosc 2009;69:253–259.
Pennazio M, Eisen G, Goldfarb N. ICCE consensus for obscure gastrointestinal bleeding. Endoscopy 2005;37:1046–1050.
de Franchis R, Avgerinos A, Barkin J, Cave D, Filoche B. ICCE consensus for bowel preparation and prokinetics. Endoscopy 2005;37:1040–1045.
Jones BH, Fleischer DE, Sharma VK, et al. Yield of repeat wireless video capsule endoscopy in patients with obscure gastrointestinal bleeding. Am J Gastroenterol 2005;100:1058–1064.
Bar-Meir S, Eliakim R, Nadler M, et al. Second capsule endoscopy for patients with severe iron deficiency anemia. Gastrointest Endosc 2004;60:711–713.
Viazis N, Papaxoinis K, Vlachogiannakos J, Efthymiou A, Theodoropoulos I, Karamanolis DG. Is there a role for second-look capsule endoscopy in patients with obscure GI bleeding after a nondiagnostic first test?. Gastrointest Endosc 2009;69:850–856.
Korman LY, Delvaux M, Gay G, et al. Capsule endoscopy structured terminology (CEST): proposal of a standardized and structured terminology for reporting capsule endoscopy procedures. Endoscopy 2005;37:951–959.
Hartmann D, Eickhoff A, Damian U, Riemann JF. Diagnosis of small-bowel pathology using paired capsule endoscopy with two different devices: a randomized study. Endoscopy 2007;39:1041–1045.
Cave DR, Fleischer DE, Leighton JA, et al. A multicenter randomized comparison of the Endocapsule and the PillCam SB. Gastrointest Endosc 2008;68:487–494.
Kimble JS, Chak A, Isenberg GA, Cooper GS, Wong RC. Variation in diagnostic yield of back-to-back capsule endoscopy in obscure GI bleeding: final results. Gastrointest Endosc 2007;65:AB185.
Rastogi A, Schoen RE, Slivka A. Diagnostic yield and clinical outcomes of capsule endoscopy. Gastrointest Endosc 2004;60:959–964.
Westerhof J, Weersma RK, Koornstra JJ. Risk factors for incomplete small-bowel capsule endoscopy. Gastrointest Endosc 2009;69:74–80.
Endo H, Matsuhashi N, Inamori M, et al. Abdominal surgery affects small bowel transit time and completeness of capsule endoscopy. Dig Dis Sci 2009;54:1066–1070.
Shim KN, Kim YS, Kim KJ, et al. Abdominal pain accompanied by weight loss may increase the diagnostic yield of capsule endoscopy: a Korean multicenter study. Scand J Gastroenterol 2006;41:983–988.
Fry LC, Carey EJ, Shiff AD, et al. The yield of capsule endoscopy in patients with abdominal pain or diarrhea. Endoscopy 2006;38:498–502.
Rondonotti E, Pennazio M, Toth E, et al. Small-bowel neoplasms in patients undergoing video capsule endoscopy: a multicenter European study. Endoscopy 2008;40:488–495.
Higuchi K, Umegaki E, Watanabe T, et al. Present status and strategy of NSAIDs-induced small bowel injury. J Gastroenterol 2009;44:879–888.
Endo H, Hosono K, Inamori M, et al. Characteristics of small bowel injury in symptomatic chronic low-dose aspirin users: the experience of two medical centers in capsule endoscopy. J Gastroenterol 2009;44:544–549.