만성 C형 간염의 미국 가이드라인 AASLD (최근 새로운 약제의 출현으로 자주 내용이 업데이트 됩니다. 최신 가이드라인은 아래링크를 클릭하여 확인 바랍니다.)
우리나라 대한간학회의 가이드라인은 미국 간학회 AASLD와 유럽간학회 EASL을 쫓아가나 국내에 보험약가가 정해지고 난 뒤에 정해지므로 치료도중 새로운 약제가 나오거나 가이드라인이 바뀔 경우 바로 치료를 시작하는 것과 새로운 약제의 출시를 기다리는 것 사이에 고려해야 할 점들이 많이 있습니다.
https://www.hcvguidelines.org/full-report-view
만성 C형간염의 초치료
HCV 유전자형 1a 환자는 어떤 치료방법으로 치료할 경우 HCV 유전자형 1b 환자보다 더 높은 재발을하는 경향이있다. 서브 타입 화 될 수 없는 유전자 1 형 HCV 감염은 일단 유전자형 1a 감염으로 취급되어야한다.
HCV 치료에 DAA의 도입은 환자에 사용되는 다른 약물과 약물 상호 작용의 위험성을 증가하고, 현재 DAA의 조합으로, 약물 상호 작용에 대한 관심이 더욱 중요해지고 있습니다. (참조 약물 상호 작용 표 ).
특히, ledipasvir (90 mg) 및 sofosbuvir (400 mg)을 매일 고정 된 용량 으로 위산분비억제제와복용시 약물 상호작용이 있을 수 있어서 PPI 등의 치료는 HCV 치료전에 하는 것이 좋습니다. HCV 치료동안 PPI 등의 치료를 하려면 최소용량등으로 용량조절이 필요할 수 있습니다.
paritaprevir (150 mg), ritonavir (100 mg) 그리고 ombitasvir (25 mg)와 dasabuvir (250 mg)을 투여하는 경우에도 흡입 베타 – 아드레날린 수용체 작용제 살 메테롤 (기관지 확장제)을 같이 투여시 상호 작용으로 심전도의 QT 간격 연장을 포함한 심혈 관계 이상 반응의 위험이 증가 하기때문에 사용하지 않는 것이 좋습니다.
For HCV genotype 1a–infected, treatment-naive patients, there are 3 regimens of comparable efficacy, as outlined above.
Ledipasvir/sofosbuvir was approved by the FDA for the treatment of HCV genotype 1 infection in treatment-naive patients based on 2 registration trials: ION-1(865 treatment-naive patients; those with cirrhosis were included) and ION-3 (647 treatment-naive patients; those with cirrhosis were excluded). ION-1 investigated length of treatment (12 weeks vs 24 weeks) and the need for RBV. (Afdhal, 2014a) Sustained virologic response at 12 weeks (SVR12) was 97% to 99% across all arms, with no difference in SVR based on length of treatment, use of RBV, or HCV genotype 1 subtype. Sixteen percent of subjects enrolled were classified as having cirrhosis. There was no difference in SVR12 in those with cirrhosis (97%) versus those without cirrhosis (98%). ION-3 excluded patients with cirrhosis and investigated shortening therapy from 12 weeks to 8 weeks (with or without RBV). (Kowdley, 2014) SVR12 was 93% to 95% across all arms, with no difference in SVR in the intention-to-treat analysis. However, relapse rates were higher in the 8-week arms (20 of 431) regardless of RBV use compared with the 12-week arm (3 of 216). Post hoc analyses of the 2 RBV-free arms assessed baseline predictors of relapse and identified lower relapse rates in patients receiving 8 weeks of ledipasvir/sofosbuvir who had baseline HCV RNA levels below 6 million IU/mL (2%; 2 of 123), and was the same for patients with similar baseline HCV RNA levels who received 12 weeks (2%; 2 of 131). This analysis was not controlled and thus substantially limits the generalizability of this approach to clinical practice. Shortening treatment to less than 12 weeks should be done with caution and performed at the discretion of the practitioner.
Paritaprevir/ritonavir/ombitasvir plus dasabuvir and weight-based RBV (1000 mg [<75 kg] to 1200 mg [>75 kg]) was approved by the FDA for the treatment of HCV genotype 1a infection in treatment-naive patients based on 3 registration trials: SAPPHIRE-I (322 treatment-naive patients with genotype 1a HCV infection; those with cirrhosis were excluded), PEARL-IV (305 treatment-naive patients with genotype 1a; those with cirrhosis were excluded), and TURQOUISE-II (261 treatment-naive and -experienced patients with genotype 1a; those with cirrhosis were included). The SAPPHIRE-I trial reported a high SVR12 rate (95.3%) with 12 weeks of paritaprevir/ritonavir/ombitasvir plus dasabuvir and RBV. (Feld, 2014) Overall, virologic failure was higher for patients with HCV genotype 1a (7 of 8) than patients with HCV genotype 1b (1 virologic failure). PEARL-IV was specifically designed to determine the role of paritaprevir/ritonavir/ombitasvir plus dasabuvir with or without weight-based RBV for treatment-naive, HCV genotype 1a–infected patients without cirrhosis. (Ferenci, 2014) SVR12 was lower in the RBV-free arm than in the RBV-containing arm (90% vs 97%, respectively) owing to higher rates of virologic failure (7.8% vs 2%, respectively), confirming the need for weight-based RBV for patients with HCV genotype 1a. TURQUOISE-II enrolled treatment-naive and -experienced patients (261 patients with HCV genotype 1a) with CTP class A cirrhosis to receive either 12 weeks or 24 weeks of treatment with paritaprevir/ritonavir/ombitasvir plus dasabuvir and RBV. Overall, SVR12 rates were 89% in the 12-week arm and 95% in the 24-week arm. (Ombitasvir/Paritaprevir/Ritonavir prescribing information); (Poordad, 2014) This difference in SVR12 rate between arms was primarily driven by patients with null response to PEG-IFN and RBV; there was less difference in SVR rates in the patients with cirrhosis who were naive to therapy (92% and 95%, respectively). (Ombitasvir/Paritaprevir/Ritonavir prescribing information); (Poordad, 2014)
COSMOS was a phase II clinical trial of sofosbuvir plus simeprevir that included treatment-naive and -experienced patients, including those with cirrhosis. (Lawitz, 2014a) The study enrolled 2 cohorts: cohort 1 included 80 patients with a prior null response to PEG-IFN and RBV with Metavir F0 to F2 fibrosis, and cohort 2 included 87 patients who were either treatment naive or had a prior null response to PEG-IFN and RBV with Metavir stage F3 or F4 fibrosis. Each cohort had 4 arms to address length of treatment (12 weeks vs 24 weeks) and the role of weight-based RBV. Across both cohorts, the SVR12 rate ranged from 79.3% to 100%, with no clear benefit of extended treatment or use of RBV; however, the small size per arm limited the analysis. In a pooled analysis of all RBV-free arms, 95% (20/21) with Metavir stage F0 to F3 fibrosis who received 12 weeks of sofosbuvir and simeprevir achieved SVR12 compared with 86% (6/7) of those with Metavir stage F4 fibrosis. (Lawitz, 2014b) Among patients with cirrhosis who received 24 weeks of therapy, the SVR rate was 100% (10/10).
Based on this analysis, the FDA recommends 24 weeks for all patients with cirrhosis, regardless of treatment experience. An ongoing phase III trial ( NCT02114151 ) will provide more definitive information on the response rates of patients with cirrhosis receiving 12 weeks of sofosbuvir and simeprevir without RBV. Until that time, the use of 24 weeks of sofosbuvir and simeprevir for patients with cirrhosis is recommended. Overall, only 6 patients in the COSMOS trial experienced virologic failure (relapse), all had HCV genotype 1a infection, and 4 had virus with the Q80K polymorphism at baseline. When simeprevir is combined with PEG-IFN and RBV, patients with HCV genotype 1a infection and a baseline Q80K polymorphism have higher virologic failure rates than patients with genotype 1b infection or genotype 1a infection without this baseline mutation. This led to the recommendation for baseline Q80K testing for all HCV genotype 1a–infected patients prior to the use of simeprevir with PEG-IFN and RBV. In contrast, the presence of the Q80K polymorphism does not preclude treatment with simeprevir and sofosbuvir, because the SVR rate was high in patients with HCV genotype 1a and the Q80K polymorphism (88%; 51/58). (Lawitz, 2014b) Given the low but finite failure rates, the role of RBV with simperevir and sofosbuvir in patients with HCV genotype 1a is unclear. Thus, RBV use may be considered until results from the larger phase III trials can more definitively address these questions (NCT02114151, NCT02114177).
The safety profiles of all the recommended regimens above are excellent. Across numerous phase III programs, less than 1% of patients without cirrhosis discontinued treatment early and adverse events were mild. Most adverse events occurred in RBV-containing arms. Discontinuation rates were higher for patients with cirrhosis (approximately 2% for some trials) but still very low.
For HCV genotype 1b–infected, treatment-naive patients, there are 3 regimens of comparable efficacy, as outlined above.
There is no measurable difference demonstrated to date in treatment response to ledipasvir/sofosbuvir for HCV genotype 1 subtypes, thus the supporting evidence remains the same as for HCV genotype 1a–infected patients (see Genotype 1).
Paritaprevir/ritonavir/ombitasvir plus dasabuvir with or without cirrhosis and RBV (cirrhosis) was approved by the FDA for the treatment of HCV genotype 1b infection in treatment-naive patients based on 3 registration trials: SAPPHIRE-I (151 treatment-naive patients with genotype 1b HCV; those with cirrhosis were excluded), PEARL-III (419 treatment-naive patients, all with genotype 1b; those with cirrhosis were excluded), and TURQOUISE-II (119 treatment-naive and -experienced patients with genotype 1b; those with cirrhosis were included). SAPPHIRE-I reported a high SVR12 rate (98%) with 12 weeks of paritaprevir/ritonavir/ombitasvir plus dasabuvir and RBV in patients with HCV genotype 1b. (Feld, 2014) Given the high SVR12 rates seen in SAPPHIRE-I, PEARL-III was specifically designed to determine the role of weight-based RBV with paritaprevir/ritonavir/ombitasvir plus dasabuvir in treatment-naive patients with HCV genotype 1b without cirrhosis. (Ferenci, 2014) SVR12 rate was 99% in both arms, confirming the lack of added benefit of weight-based RBV for patients without cirrhosis with HCV genotype 1b. TURQUOISE-II enrolled treatment-naive and -experienced patients with CTP class A cirrhosis to receive either 12 weeks or 24 weeks of treatment with paritaprevir/ritonavir/ombitasvir plus dasabuvir and RBV. Overall, SVR12 rates were 98.5% in the 12-week arm and 100% in the 24-week arm. (Poordad, 2014) Owing to the high SVR rates, a phase III study is currently underway to determine if RBV is required as part of this regimen for HCV genotype 1b–infected patients with cirrhosis (TURQUOISE-III, NCT02219503). Until these data are available, treatment-naive patients with HCV genotype 1b infection and cirrhosis should receive RBV with paritaprevir/ritonavir/ombitasvir plus dasabuvir.
As noted, COSMOS was a phase II clinical trial of sofosbuvir plus simeprevir with or without weight-based RBV for 12 weeks or 24 weeks. (Lawitz, 2014a) The study enrolled 2 cohorts: cohort 1 included 80 patients with a prior null response to PEG-IFN and RBV with Metavir stage F0 to F2 fibrosis and cohort 2 included 87 patients who were treatment naive or who had a prior null response to PEG-IFN and RBV with Metavir stage F3 or F4 fibrosis. No virologic failure occurred in patients with HCV genotype 1b infection, suggesting high efficacy of this regimen for this genotype. Preliminary data from large prospective observational cohort studies demonstrate high SVR rates (>90%) in patients with HCV genotype 1b infection treated with sofosbuvir plus simeprevir in clinical practice. These data provide additional support for use of this DAA combination for the treatment of HCV genotype 1b. (Jensen, 2014); (Dieterich, 2014a) The FDA approval of this combination recommends 24 weeks of the combination without RBV in all patients with cirrhosis. Phase III study data of this regimen should be available in mid-2015 and are expected to provide further insight into the treatment of patients with HCV genotype 1b, including the need for RBV and optimal treatment duration for patients with cirrhosis (NCT02114151, NCT02114177).
The safety profiles to date of all recommended regimens above are excellent. Across numerous phase III programs, less than 1% of patients without cirrhosis discontinued treatment early and adverse events were mild. Most adverse events occurred in RBV-containing arms. Discontinuation rates were higher for patients with cirrhosis (approximately 2% for some trials) but still very low.
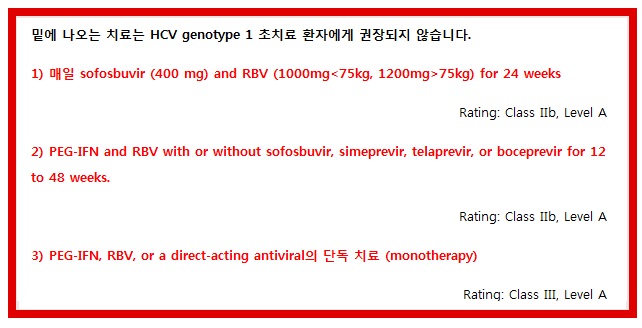
밑에 나오는 치료는 HCV genotype 1 초치료 환자에게 권장되지 않습니다.
*See sections on HIV/HCV coinfection, decompensated cirrhosis, liver transplantation, and renal impairment.
Although regimens of sofosbuvir and RBV or PEG-IFN and RBV plus sofosbuvir, simeprevir, telaprevir, or boceprevir for 12 weeks to 48 weeks (some using response-guided therapy) are also FDA approved, they are inferior to the current recommended regimens. Most of the IFN-containing regimens are associated with higher rates of serious adverse events (eg, anemia and rash), longer treatment duration, high pill burden, numerous drug-drug interactions, more frequent dosing, higher intensity of monitoring for continuation and stopping of therapy, and the requirement to be taken with food or with high-fat meals. Although the phase III NEUTRINO trial reported the highest SVR rate (89%) for an IFN-containing regimen (sofosbuvir [400 mg daily]) in combination with PEG-IFN 2a (180 μg by subcutaneous injection weekly) and weight-based RBV (1000 mg [<75 kg] to 1200 mg [≥75 kg]) in HCV genotype 1 infection and limited exposure to IFN to just 12 weeks, the safety and tolerability profile limits its usefulness in the setting of FDA-approved, highly efficacious oral DAA combinations. (Lawitz, 2013a)
PEG-IFN and RBV for 48 weeks for treatment-naive patients with HCV genotype 1 has been superseded by treatments incorporating DAAs and should not be used.
Sofosbuvir (400 mg daily) was combined with weight-based RBV (1000 mg [<75 kg] to 1200 mg [≥75 kg]) for treatment-naive patients with HCV genotype 2 infection in 3 clinical trials, each of which enrolled patients with genotype 2 or 3 HCV: FISSION, POSITRON, and VALENCE. (Lawitz, 2013a); (Jacobson, 2013c); (Zeuzem, 2013c) The FISSION study randomized patients to receive daily PEG-IFN and RBV (800 mg) for 24 weeks or sofosbuvir plus daily weight-based RBV (1000 mg [<75 kg] to 1200 mg [≥75 kg]) for 12 weeks. (Lawitz, 2013a) The SVR rate was higher (94%) in patients who received sofosbuvir plus RBV than in those who received PEG-IFN and RBV (78%; 52/67). Across all 3 trials, 201 of the 214 (94%) patients with HCV genotype 2 achieved SVR with sofosbuvir plus RBV. Among patients who did not achieve SVR, sofosbuvir resistance–associated variants (RAVs) were not detected. (US FDA, 2013a) Based on real-world data from Trio Health, lower response rates were seen in treatment-naive patients with cirrhosis than in those without cirrhosis. (Dieterich, 2014a) Although data to support extension of therapy are not yet available for treatment-naive patients with HCV genotype 2 infection, longer treatment duration improves SVR in treatment-experienced patients with cirrhosis. (Jacobson, 2013c) Owing to the small numbers of patients with HCV genotype 2 infection and cirrhosis enrolled in the registration trials, several phase IIIB studies are ongoing to specifically determine the appropriate length of treatment for this subgroup of patients (NCT01962441, NCT 02128542). Until these data are available, extending treatment from 12 weeks to 16 weeks in HCV genotype 2–infected patients with cirrhosis is recommended.
Alternative regimens for treatment-naive patients with HCV genotype 2 infection.
None.
Although there are no alternative regimens listed, several available DAAs have activity in vitro and in vivo against HCV genotype 2. Simeprevir has moderate potency against HCV genotype 2 but has not formally been tested in combination with sofosbuvir in HCV genotype 2 infection. (Moreno, 2012) Daclatasvir (60 mg/day) with sofosbuvir (400 mg/day) for 24 weeks was associated with high rates of SVR in treatment-naive patients with HCV genotype 2 infection. Although the 50% effective concentration (EC50) for daclatasvir increases several logs in the presence of the prevalent M31 polymorphism, the drug maintains adequate activity against HCV genotype 2. (Wang, 2014) Although approved by regulatory authorities in some regions, daclatasvir is not approved by the FDA for use in the United States at this time. For patients who require treatment but cannot tolerate RBV, a discussion with a practitioner with expertise in the treatment of HCV infection is recommended prior to the use of approved RBV-free DAA combinations for HCV genotype 2 infection.
PEG-IFN 2a (180 µg weekly) or PEG-IFN 2b (1.5 µg/kg weekly) plus RBV (800 mg daily) for 24 weeks was compared with sofosbuvir (400 mg daily) plus weight-based RBV (1000 mg [<75 kg] to 1200 mg [≥75 kg]) in the FISSION trial. (Lawitz, 2013a) The SVR12 rate achieved with PEG-IFN and RBV was lower than that achieved with sofosbuvir and RBV overall (78% and 95%, respectively) and in the subgroups of patients with or without cirrhosis. Safety and tolerability of PEG-IFN and RBV was inferior to that observed with sofosbuvir and RBV, with greater frequency of reported adverse events and laboratory abnormalities and a higher rate of treatment discontinuation owing to adverse events. Further, therapy with PEG-IFN and RBV is 12 weeks longer than that with sofosbuvir plus RBV.
Because of its poor activity in vitro and in vivo, boceprevir should not be used as therapy for patients with HCV genotype 2 infection. Although telaprevir plus PEG-IFN and RBV has antiviral activity against HCV genotype 2, (Foster, 2011) the additional adverse effects and longer duration of therapy required do not support use of this regimen. Similarly, although ledipasvir has adequate activity against HCV genotype 2, this is lost in the presence of the highly prevalent M31 polymorphism and thus is not recommended for treatment of HCV genotype 2 infection. (Nakamoto, 2014)
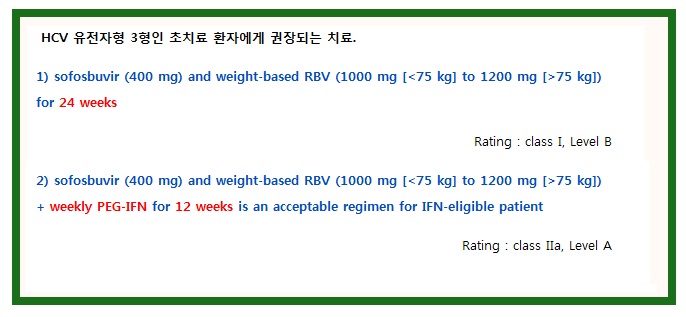
The VALENCE study, which enrolled patients with genotype 2 or 3 HCV infection, assessed the efficacy and safety of sofosbuvir (400 mg daily) plus weight-based RBV for 24 weeks. This trial included 250 treatment-naive (42%) and -experienced (58%) subjects with HCV genotype 3 infection. The overall SVR12 rate was 84% and was higher among treatment-naive than -experienced patients (93% vs 77%, respectively). (Zeuzem, 2014) These results suggest that higher response rates can be achieved with a 24-week duration of sofosbuvir plus RBV than those reported for HCV genotype 3–infected participants receiving 12- or 16-week regimens in the FISSION (Lawitz, 2013a) (12 weeks, SVR12 rate: 63%), POSITRON, (Jacobson, 2013c) (12 weeks, SVR 12 rate: 61%) and FUSION (12 weeks, SVR12 rate: 30%; 16 weeks, SVR12 rate: 62%) trials. The primary reason for the higher SVR rate with extended therapy among treatment-naive patients was a reduction in the relapse rate from 40% to 5%. In a subanalysis, response rates were similarly high among those with (n=45) and without (n=100) cirrhosis (92% and 93%, respectively).
The combination of sofosbuvir plus PEG-IFN and RBV was evaluated in patients with HCV genotype 3 infection. In 2 phase II clinical trials, PROTON and ELECTRON, 38 of 39 (97%) treatment-naive patients with HCV genotype 3 infection achieved SVR with sofosbuvir plus PEG-IFN (4-12 weeks of therapy) and RBV. (Gane, 2013b) For many patients with HCV genotype 3 infection, the adverse effects and increased monitoring requirements of PEG-IFN make this less acceptable than the recommended regimen of sofosbuvir plus weight-based RBV. However, the shortened treatment period may be of interest to some.
Although the combination of PEG-IFN and RBV is an FDA-approved regimen for HCV genotype 3 infection, its less acceptable adverse effect profile, need for more intensive monitoring, and overall lower efficacy make it less desirable than the recommended regimen.
Because of their limited in vitro and in vivo activity against HCV genotype 3, boceprevir, telaprevir, and simeprevir should not be used as therapy for patients with HCV genotype 3 infection.
Very limited phase II data are available from a single-center study (ELECTRON-II), which examined ledipasvir/sofosbuvir with (n=26) or without (n=25) RBV for 12 weeks in treatment-naive patients with HCV genotype 3 infection, 15% of whom had cirrhosis. All 26 (100%) patients in the RBV-containing arm achieved SVR12 compared with 16 of 25 (64%) of those in the RBV-free arm. Although these data raise the possibility that the addition of ledipasvir to sofosbuvir and ribavirin may shorten the course of therapy for persons with HCV genotype 3 infection, the high EC50 of ledipasvir for HCV genotype 3 (Wong, 2013); (Kohler, 2014) and the homogenous patient population studied limit the generalizability of this study. Until further data are available to confirm these findings, a recommendation for use of this regimen cannot be made at this time. (Gane, 2013b)
ALLY 3 is a phase III study of the investigational once-daily nonstructual protein 5A (NS5A) inhibitor daclatasvir (60 mg) plus sofosbuvir (400 mg) for 12 weeks; the study included 101 treatment-naive patients and demonstrated an SVR12 rate of 90%. In treatment-naive patients without cirrhosis (Metavir F0-F3), 97% achieved SVR12, and in treatment-naive patients with cirrhosis (Metavir F4), 58% achieved SVR12. (Nelson, 2014) Although approved by regulatory authorities in some regions, daclatasvir is not available for use in the United States at this time.
Three options with similar efficacy in general are recommended for treatment-naive patients with HCV genotype 4 infection (listed in alphabetic order; see text).
Daily fixed-dose combination of ledipasvir (90 mg)/sofosbuvir (400 mg) for 12 weeks is recommended for treatment-naive patients with HCV genotype 4 infection.
Rating: Class IIb, Level B
Daily fixed-dose combination of paritaprevir (150 mg)/ritonavir (100 mg)/ombitasvir (25 mg) and weight-based RBV (1000 mg [<75 kg] to 1200 mg [>75 kg]) for 12 weeks is recommended for treatment-naive patients with HCV genotype 4 infection.
Rating: Class I, Level B
Daily sofosbuvir (400 mg) and weight-based RBV (1000 mg [<75 kg] to 1200 mg [>75 kg]) for 24 weeks is recommended for treatment-naive patients with HCV genotype 4 infection.
Rating: Class IIa, Level B
PEARL-I was an open-label phase IIb study that included a cohort of 86 treatment-naive patients with HCV genotype 4 infection with or without cirrhosis who received 12 weeks of the daily fixed-dose combination of paritaprevir/ritonavir/ombitasvir with or without weight-based RBV. SVR12 rates were 100% (42/42) in the group receiving RBV and 90.9% (40/44) in the group not receiving RBV. Adverse effects were generally mild, with headache, asthenia, fatigue, and nausea most commonly reported. There were no discontinuations owing to adverse events. (Pol, 2014)
Several studies support the use of sofosbuvir and RBV in treatment-naive patients with HCV genotype 4 infection. In a small study of Egyptian patients in the United States treated with sofosbuvir plus weight-based RBV (1000 mg [<75 kg] to 1200 mg [>75 kg]), SVR12 was achieved in 79% (11/14) and 100% (14/14) of treatment-naive patients treated for 12 weeks and 24 weeks, respectively. (Ruane, 2014) In a phase II study in Egypt, patients with HCV genotype 4 infection received daily sofosbuvir plus weight-based RBV (1000 mg [<75 kg] to 1200 mg [≥75 kg]) for 12 weeks or 24 weeks; among treatment-naive patients, SVR12 rates were 84% (21/25) and 92% (22/24), respectively. (Esmat, 2014) PHOTON-2, an open-label study of HIV/HCV-coinfected patients, included 31 treatment-naive patients with HCV genotype 4 infection who received daily sofosbuvir plus weight-based RBV (1000 mg [<75 kg] to 1200 mg [≥75 kg]) for 24 weeks. In this study, 84% of patients (26/31) achieved SVR12. (Molina, 2014)
The SYNERGY trial is an open-label study evaluating 12 weeks of ledipasvir/sofosbuvir in 21 HCV genotype 4–infected patients, of whom 60% were treatment-naive and 43% had advanced fibrosis (Metavir F3 or F4). One patient took the first dose and then withdrew consent. In an interim analysis in which 20 patients had completed posttreatment week 12 follow-up, SVR12 rate was 95% by intention-to-treat analysis and 100% by per-protocol analysis. (Kapoor, 2014)
Alternative regimens for treatment-naive patients with HCV genotype 4 infection.
Daily sofosbuvir (400 mg) and weight-based RBV (1000 mg [<75 kg] to 1200 mg [>75 kg]) plus weekly PEG-IFN for 12 weeks is an acceptable regimen for treatment-naive patients with HCV genotype 4 infection.
Rating: Class II, Level B
Daily sofosbuvir (400 mg) plus simeprevir (150 mg) with or without weight-based RBV (1000 mg [<75 kg] to 1200 mg [>75 kg]) for 12 weeks is an acceptable regimen for treatment-naive patients with HCV genotype 4 infection.
Rating: Class IIb, Level B
In the phase III NEUTRINO trial, (Lawitz, 2013a) 28 treatment-naive patients with HCV genotype 4 infection were treated with sofosbuvir (400 mg daily) plus PEG-IFN 2a (180 µg weekly) and weight-based RBV (1000 mg [<75 kg] to 1200 mg [≥75 kg]) for 12 weeks. Of the 28 patients with HCV genotype 4 infection, 27 (96%) achieved SVR12. The single patient who did not achieve SVR had cirrhosis and had a relapse after therapy. The adverse event profile was similar to that associated with PEG-IFN and RBV therapy.
There are limited clinical data to date to support the use of the combination of 12 weeks of daily sofosbuvir (400 mg) plus simeprevir (150 mg) with or without weight-based RBV (1000 mg [<75kg] to 1200 mg [≥75mg]) in HCV genotype 4–infected patients, although studies are planned. Given the demonstrated activity of simeprevir in vitro and in vivo against HCV genotype 4, this combination may be considered as an alternative regimen. The open-label phase III RESTORE trial assessed the efficacy of simeprevir in combination with PEG-IFN and RBV in 107 patients with HCV genotype 4 infection, including 35 treatment-naive patients. In these treatment-naive patients, daily simeprevir (150 mg) for 12 weeks in combination with PEG-IFN and RBV for 24 weeks to 48 weeks (by response-guided therapy) produced an SVR in 83% (29 of 35). (Moreno, 2013a) These results are comparable to SVR rates observed with similar regimens in patients with HCV genotype 1 infection, suggesting that efficacy of sofosbuvir plus simeprevir for HCV genotype 4 infection may be roughly in line with the SVR rates of patients with HCV genotype 1 infection shown in the COSMOS trial. This combination has been approved in Europe for patients with HCV genotype 4 infection but is not FDA-approved for use in the United States.
The following regimens are NOT recommended for treatment-naive patients with HCV genotype 4 infection.
- PEG-IFN and RBV with or without simeprevir for 24 weeks to 48 weeksRating: Class IIb, Level A
- Monotherapy with PEG-IFN, RBV, or a direct-acting antiviralRating: Class III, Level A
- Telaprevir- or boceprevir-based regimensRating: Class III, Level A
PEG-IFN and RBV for 48 weeks was the previously recommended regimen for patients with HCV genotype 4 infection. (Ghany, 2009); (AASLD/IDSA/IAS-USA, 2014) Adding sofosbuvir (400 mg daily) to PEG-IFN and RBV increases response rates and markedly shortens therapy with no apparent additional adverse effects. The addition of simeprevir to PEG-IFN and RBV increases response rates but has inferior SVR rates to the other available regimens and requires a longer duration of PEG-IFN and RBV, which increases the risk of adverse events and thus is no longer recommended. (Moreno, 2013b)
Because of their limited activity against genotype 4 HCV in vitro and in vivo, boceprevir and telaprevir should not be used as therapy for patients with HCV genotype 4 infection.
Few data are available to help guide decision making for patients infected with HCV genotype 5 or 6. Nonetheless, for those patients for whom immediate treatment is required, the following recommendations have been drawn from available data.
Recommended regimen for treatment-naive patients with HCV genotype 5 infection.
Daily sofosbuvir (400 mg) and weight-based RBV (1000 mg [<75 kg] to 1200 mg [>75 kg]) plus weekly PEG-IFN for 12 weeks is recommended for treatment-naive patients with HCV genotype 5 infection.
Rating: Class IIa, Level B
In the phase III NEUTRINO trial, (Lawitz, 2013a) treatment-naive patients with HCV genotypes 1 (n=291), 4 (n=28), 5 (n=1), and 6 (n=6) were treated with sofosbuvir (400 mg daily) plus PEG-IFN 2a (180 µg weekly) and weight-based RBV (1000 mg [<75 kg] to 1200 mg [≥75 kg]) for 12 weeks. All 6 patients with HCV genotype 6 and the 1 patient with HCV genotype 5 achieved SVR12. The adverse event profile in these patients and in the larger study population was similar to that seen with PEG-IFN and RBV therapy.
Alternative regimen for treatment-naive patients with HCV genotype 5 infection.
Weekly PEG-IFN plus weight-based RBV (1000 mg [<75 kg] to 1200 mg [>75 kg]) for 48 weeks is an alternative regimen for IFN-eligible, treatment-naive patients with HCV genotype 5 infection.
Rating: Class IIb, Level A
PEG-IFN and RBV for 48 weeks was the previously recommended regimen for patients infected with HCV genotype 5, but the availability of recommended regimens that substantially reduce exposure to IFN and RBV make this regimen less appealing.
Recommended regimen for treatment-naive patients with HCV genotype 6 infection.
Daily fixed-dose combination of ledipasvir (90 mg)/sofosbuvir (400 mg) for 12 weeks is recommended for treatment-naive patients with HCV genotype 6 infection.
Rating: Class IIa, Level B
Ledipasvir has in vitro activity against most HCV genotype 6 subtypes (exception 6e). (Wong, 2013); (Kohler, 2014) A small, 2-center, open-label study (NCT01826981) investigated the safety and in vivo efficacy of ledipasvir/sofosbuvir for 12 weeks in treatment-naive and -experienced patients with HCV genotype 6 infection. Twenty-five patients (92% treatment naive) who were primarily Asian (88%) had infection from 7 different subtypes (32%, 6a; 24%, 6e; 12%, 6l; 8%, 6m; 12%, 6p; 8%, 6q; 4%, 6r). Two patients (8%) had cirrhosis. The SVR12 rate was 96% (24/25), and the 1 patient who experienced relapse had discontinued therapy at week 8 because of drug use. No patient discontinued treatment owing to adverse events.
Alternative regimen for treatment-naive patients with HCV genotype 6 infection.
Daily sofosbuvir (400 mg) and weight-based RBV (1000 mg [<75 kg] to 1200 mg [>75 kg]) plus weekly PEG-IFN for 12 weeks is an alternative regimen for IFN-eligible, treatment-naive patients with HCV genotype 6 infection.
Rating: Class IIa, Level B
In the phase III NEUTRINO trial, (Lawitz, 2013a) treatment-naive patients with HCV genotypes 1 (n=291), 4 (n=28), 5 (n=1), and 6 (n=6) were treated with sofosbuvir (400 mg daily) plus PEG-IFN 2a (180 µg weekly) and weight-based RBV (1000 mg [<75 kg] to 1200 mg [≥75 kg]) for 12 weeks. All 6 patients with HCV genotype 6 and the 1 patient with HCV genotype 5 achieved SVR12. The adverse event profile in these patients and in the larger study population was similar to that seen with PEG-IFN and RBV therapy.
The following regimens are NOT recommended for treatment-naive patients with HCV genotype 5 or 6 infection.
- Monotherapy with PEG-IFN, RBV, or a direct-acting antiviralRating: Class III, Level A
- Telaprevir- or boceprevir-based regimensRating: Class III, Level A
Because of their limited activity against genotypes 5 and 6 HCV in vitro and in vivo, boceprevir and telaprevir should not be used as therapy for patients with HCV genotype 5 or 6 infection.
Mixed Genotypes
Rarely, genotyping assays may indicate the presence of a mixed infection (eg, genotypes 1a and 2). Treatment data for mixed genotypes with direct-acting antivirals are sparse, and awaiting availability of a pangenotypic regimen may be considered. Until then, when treatment is necessary, the choice of antiviral combination and duration of treatment should maximize efficacy against each genotype represented in the assay. When the correct combination or duration is unclear, expert consultation should be sought.
Initial Treatment Table: Drug Interactions With Direct-Acting Antivirals and Selected Concomitant Medications
| Concomitant Medications | Ledipasvir | Paritaprevir / Ritonavir / Ombitasvir + Dasabuvir | Simeprevir | Sofosbuvir |
| Acid-reducing agents* | X | X | ||
| Alfuzosin/tamsulosin | X | |||
| Amiodarone | X** | X | ||
| Anticonvulsants | X | X | X | X |
| Antiretrovirals* | Coming Soon | Coming Soon | Coming Soon | tipranavir / ritonavir only |
| Azole antifungals* | X | X | ||
| Buprenorphine/naloxone | X | |||
| Calcineurin inhibitors* | X | X | ||
| Calcium channel blockers* | X | X | ||
| Cisapride | X | X | ||
| Digoxin | X | X | ||
| Ergot derivatives | X | |||
| Ethinyl estradiol–containing products | X | |||
| Furosemide | X | |||
| Gemfibrozil | X | |||
| Glucocorticoids | X (inhaled, intranasal | X | ||
| Herbals St. John’s wort Milk thistle | X | X X | X | |
| Macrolide antimicrobials* | X | |||
| Other antiarrythmics* | X | X | ||
| Phosphodiesterase type 5 inhibitors* | X | X | ||
| Pimozide | X | |||
| Rifamycin antimicrobials* | X | X | X | X |
| Salmeterol | X | |||
| Sedatives* | X | X | ||
| Simeprevir | X | |||
| Statins* | X | X | X |
*Some drug interactions are not class specific; see product prescribing information for specific drugs within a class.
**As coformulated with sofosbuvir.
Complete revision made on this section on December 19, 2014. Additional changes made on April 8, 2015
유럽 가이드라인은 EASL 참고 https://www.easl.eu/