미국 간학회 C형 간염 치료 가이드라인 소개 AASLD
텔라프레비어와 보세프레비어는 사용중지를 권유 하고 있고 소포스부비어는 가이드라인에 다 들어가 있네요.
치료기간도 유전자형 1형, 유전자형 2,3형 모두 12주로 줄어들었습니다.
우리나라에 소포스부비어 제제가 빨리 들어오길 바랍니다.
INITIAL TREATMENT OF HCV INFECTION IN PATIENTS STARTING TREATMENT
A summary of recommendations for initial treatment is found in the box.
This section provides guidance on the recommended initial treatments for persons with chronic HCV infection who are naive to HCV treatment or who have achieved an undetectable level of virus during a prior treatment course of PEG/RBV and relapsed (relapsers). Although PEG/RBV relapsers are being retreated, their treatment recommendations are presently the same as for persons being treated for the first time as described below. This section assumes that a decision to treat has been made and provides guidance regarding optimal treatment. In many instances, however, it may be advisable to delay treatment for some patients with documented early fibrosis stage (F 0-2), because waiting for future highly effective, pangenotypic, DAA combinations in IFN-free regimens may be prudent. Potential advantages of waiting to begin treatment will be provided in a future update to this guidance.
The level of evidence available to inform the best treatment decisions for each patient varies, as does the strength of the recommendation, and is graded accordingly (see Methods Table 2). In addition, when treatment differs for a particular group, such as those infected with specific HCV genotypes, specific recommendations are given. A regimen is classified as either “Recommended” when it is favored for most patients or “Alternative” when optimal in a particular subset of patients in that category. When a treatment is clearly inferior or is deemed harmful, it is classified as “Not Recommended.” Unless otherwise indicated, such regimens should not be administered to patients with HCV infection. Specific considerations of persons with HIV/HCV coinfection, compensated and decompensated cirrhosis (moderate or severe hepatic impairment; CTP class B or C), post-liver transplant HCV, and those with severe renal impairment or ESRD are addressed in other sections of the document.
As always, patients receiving antiviral therapy require careful pretreatment assessment for comborbidities that may influence treatment response. All patients should have careful monitoring during treatment, particularly for anemia if ribavirin is included in the regimen.
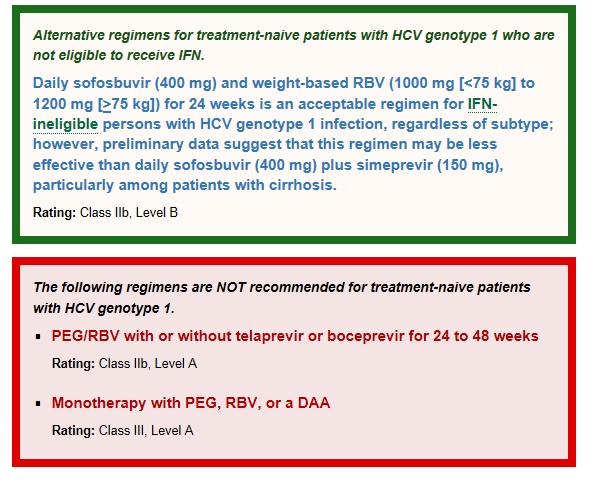
미국 간학회에서는 위의 빨간 박스 내용처럼텔라프레비어와 보세프레비어는 포함되던 포함되지 않던 상관없이 페그인터페론과 리바비린치료 24 -48주 치료는 권고되지 않습니다.
페그인터페론, 리바비린, DAA 제제의 단독요법도 권고 하지 않습니다.
표준 치료는 매일 소포스부비어(400mg)와 체중에 따른 리바비린 (1000-1200mg)과 함께 페그인터페론 12주를 권고하고 있습니다.
Sofosbuvir is a prodrug of a nucleotide analogue inhibitor of the HCV NS5B RNA-dependent RNA polymerase. The phase 3 NEUTRINO trial evaluated sofosbuvir (400 mg daily) in combination with PEG (2a) (180 μg by subcutaneous injection weekly) and weight-based RBV (1000 mg to 1200 mg daily) for 12 weeks in 291 treatment-naive patients with chronic HCV genotype 1 infection. (Lawitz, 2013b) The SVR12 for patients with genotype 1 infection was 89%. SVR12 did not differ substantially by baseline characteristic but was lower in patients with cirrhosis (80%) than in those without cirrhosis (92%). (Lawitz, 2013b)
COSMOS is an ongoing phase 2 clinical trial of sofosbuvir (400 mg daily) plus simeprevir (150 mg daily), a specific inhibitor of the HCV NS3/4A serine protease, with or without RBV for 12 or 24 weeks. (Jacobson, 2013b) The study enrolled 2 cohorts: cohort 1 included patients with a prior null response to PEG/RBV with Metavir fibrosis stage of 0 or 2 (n=80); Cohort 2 included patients who were either treatment-naive or had a prior null response with Metavir fibrosis stage of 3 or 4 (n=87). In cohort 1, the 12-week treatment groups, SVR12 was 96% and 93% in patients treated with or without RBV, respectively. The 24-week treatment groups had SVR12 of 79.3% and 93% in patients treated with or without RBV, respectively. No viral breakthrough was observed in cohort 1 during treatment, and 3 patients experienced viral relapse after stopping therapy. All 3 patients with viral relapse were infected with HCV genotype 1a and had the Q80K polymorphism.
Preliminary SVR4 results are available for cohort 2. The 12-week treatment duration group had 100% SVR in treatment-naive patients treated with or without RBV, and 100% and 93.3% in prior null responder patients treated with or without RBV, respectively. No viral breakthrough was observed during treatment; 1 patient infected with HCV genotype 1a/Q80K experienced viral relapse after stopping therapy. No SVR data are yet available from cohort 2, which received 24 weeks of treatment.
Among patients who had viral relapse, simeprevir (protease) resistance-associated variants have been observed; sofosbuvir (polymerase) resistance-associated variants have not been detected. Safety data have been presented for all 167 patients treated. The combination was well tolerated, with only 2.4% of patients prematurely discontinuing therapy due to adverse events. Data on the use of simeprevir in patients with hepatic impairment are not available at this time.
For patients infected with genotype 1a HCV, baseline resistance testing for the Q80K polymorphism may be considered. However, in contrast to using simeprevir to treat a genotype 1a HCV patient with PEG/RBV when the mutation markedly alters the probability of an SVR, the finding of the Q80K polymorphism does not preclude treatment with simeprevir and sofosbuvir, because the SVR rate was high in patients with genotype 1a/Q80K infection (SVR12 rate for cohort 1 was 86% [24 of 28 patients]; SVR4 rate for cohort 2 was 90% [10 of 11 patients]). To date, virologic failure has not been observed in patients in either cohort infected with HCV genotype 1b and with HCV genotype 1a in the absence of the Q80K polymorphism. Thus Q80K testing can be considered but is not strongly recommended.
This regimen should be considered only in those patients who require immediate treatment, because it is anticipated that safer and more effective IFN-free regimens will be available by 2015.
Two randomized, placebo-controlled phase 3 trials evaluated the efficacy and safety of simeprevir (150 mg once daily) for 12 weeks plus PEG and weight-based RBV for a total of 24 weeks (RGT design found no advantage to extending PEG/RBV to 48 weeks). (Jacobson, 2013a); (Poordad, 2013)
In both studies, SVR24 rates were significantly higher among the simeprevir-containing arms (80% to 81%) than in the non-simeprevir-containing arms (50%). If the HCV RNA at week 4 of treatment is less than 25 IU/mL, therapy should be continued to week 24. If the HCV RNA is greater than 25 IU/mL at treatment week 4 or any treatment week thereafter, the regimen should be discontinued. In patients with HCV genotype 1a infection, the presence of a naturally occurring NS3-4A protease polymorphism (Q80K) prior to treatment was associated with a substantial reduction in SVR among patients treated with simeprevir. A statistically significant difference in SVR12 rates exists between simeprevir-treated persons who are infected with HCV genotype 1a but do not have the Q80K polymorphism and placebo-treated patients who likewise have no such polymorphism. This difference was noted in both the pooled treatment-naive studies and the relapser study (SVR rates of 84% versus 43%, respectively [treatment-naive study] and 78% versus 24%, respectively [relapse study]). The overall SVR in the subgroup of patients with baseline Q80K polymorphism was no better than that in the placebo group. In the United States, persons with genotype 1a HCV infection have a high prevalence of Q80K polymorphism. Because these persons may require alternative therapy, baseline testing for Q80K is recommended for all patients before treatment with the simeprevir plus PEG/RBV regimen is initiated.
For the simeprevir plus PEG/RBV treatment regimen, if the HCV RNA at week 4 of treatment is less than 25 IU/mL, therapy should be continued to week 24. If the HCV RNA is greater than 25 IU/mL at treatment week 4 or any treatment week thereafter, the regimen should be discontinued.
Sofosbuvir plus RBV was evaluated in 60 treatment-naive patients with HCV genotype 1 with unfavorable treatment characteristics (eg, African American race and advanced fibrosis). (Osinusi, 2013) In part 1 of the study, 10 participants with early to moderate liver fibrosis were treated with sofosbuvir (400 mg daily) plus weight-based RBV for 24 weeks. Nine participants (90%) achieved SVR24. In part 2, 50 participants with any stage of liver fibrosis were randomized 1:1 to receive 400 mg sofosbuvir with RBV either weight-based or low-dose (600 mg daily) for 24 weeks; SVR24 was 68% (17/25) in the weight-based group and 48% (12/25) in the low-dose group. The regimens used in part 2 of this study were well tolerated, with no discontinuations due to adverse events. Seven of the 13 participants (54%) with advanced liver fibrosis treated in this study relapsed, including all 4 with cirrhosis.
Several additional studies have evaluated the effectiveness of sofosbuvir in persons with HCV genotype 1. In the QUANTUM trial, 38 treatment-naive patients with HCV genotype 1 who did not have cirrhosis were assigned either 12 (n=19) or 24 (n=19) weeks of sofosbuvir (400 mg daily) and weight-based RBV. (Lalezari, 2013) Ten of 19 (53%) in the 12-week arm and 9 of 19 (47%) subjects in the 24-week arm achieved SVR12 (overall 50%). In the ELECTRON trial, 25 treatment-naive subjects with HCV genotype 1 who did not have cirrhosis received sofosbuvir plus RBV for 12 weeks. Twenty-one (84%) achieved SVR12. (Gane, 2013b) In the PHOTON-1 trial, 86 of 113 (76%) treatment-naive subjects with genotype 1 HCV/HIV coinfection achieved SVR12 with sofosbuvir plus RBV for 24 weeks. (Sulkowski, 2013c) Taken together, in a total of 211 subjects, the range of SVR for regimens incorporating sofosbuvir plus daily weight-based RBV (1000 mg to 1200 mg) for up to 24 weeks in treatment-naive persons with HCV genotype 1 was 50% to 84%, with an overall SVR of 72%. Sofosbuvir resistance-associated amino acid variants have not been detected among those patients treated with this combination who did not achieve SVR.
This regimen should be considered only in those patients who require immediate treatment. It is estimated that the FDA will approve safer and more effective IFN-free regimens by 2015.
Although regimens of PEG/RBV plus telaprevir or boceprevir for 24 to 48 weeks using RGT are also FDA approved, they are markedly inferior to the preferred and alternative regimens. These regimens are associated with their higher rates of serious adverse events (eg, anemia and rash), longer treatment duration, high pill burden, numerous drug-drug interactions, frequency of dosing, intensity of monitoring for continuation and stopping of therapy, and the requirement to be taken with food or with high-fat meals.
PEG/RBV for 48 weeks for treatment-naive subjects with HCV genotype 1 has been superseded by treatments incorporating DAAs and should not be used.
II. Genotype 2
유전자 2형에서도 텔라프레비어와 보세프레비어, 시메프레비어 베이스의 치료레지멘 모두 권고하지 않습니다.
다른 대체할만한 치료 레지멘은 없다고 표현되어 있네요. 무조건 데일리 소포스부비어 400mg과 체중에 따른 리바비린 12주 요법이 표준 치료법으로 권고하고 있습니다.
Sofosbuvir (400 mg daily) was combined with weight-based RBV (1000 mg to 1200 mg) to treat HCV genotype 2 treatment-naive patients across 3 clinical trials: FISSION, POSITRON, and VALENCE. (Lawitz, 2013b); (Jacobson, 2013c); (Zeuzem, 2013b) The FISSION study randomized patients to daily PEG/RBV (800 mg) for 24 weeks or sofosbuvir plus daily weight-based RBV (1000 mg to 1200 mg). (Lawitz, 2013b) The SVR was higher (94%) in patients who received sofosbuvir plus RBV compared with those who received PEG/RBV (78%) (52/67). Across all 3 trials, 201 of 214 (94%) patients with HCV genotype 2 achieved SVR with sofosbuvir plus RBV. Among patients who did not achieve SVR, sofosbuvir resistance-associated amino acid variants were not detected. (US FDA, 2013a)
Alternative Regimens for treatment-naive patients with genotype 2:
None
PEG (2a) (180 µg weekly) or PEG (2b) (1.5 µg/kg weekly) plus RBV (800 mg daily) for 24 weeks was directly compared with sofosbuvir (400 mg daily) plus weight-based RBV (1000 mg to 1200 mg daily) in the FISSION trial. (Lawitz, 2013b) The SVR12 achieved with PEG/RBV was lower than that achieved with sofosbuvir/RBV overall (78% and 95%, respectively) and in the subgroups of patients with or without cirrhosis. Safety and tolerability of PEG/RBV was inferior to the profile observed with sofosbuvir and RBV, with greater frequency of reported adverse events and laboratory abnormalities as well as a higher rate of treatment due to adverse events. Further, the duration of therapy with PEG/RBV is 12 weeks longer than that of sofosbuvir plus RBV.
Due to their poor in vitro and in vivo activity, boceprevir and simeprevir should not be used as therapy for patients with HCV genotype 2 infection. Although telaprevir combined with PEG/RBV has antiviral activity against HCV genotype 2, (Foster, 2011) the additional side effects and longer duration of therapy do not support use of this regimen.
III. Genotype 3
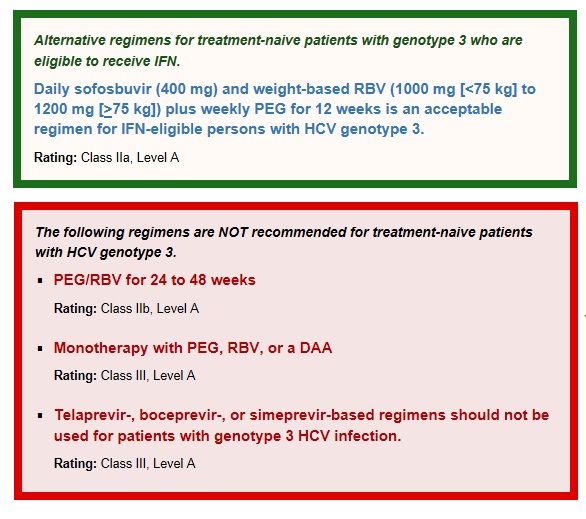
The VALENCE study assessed the efficacy and safety of sofosbuvir (400 mg daily) plus RBV for 24 weeks in 250 treatment-naive (42%) and treatment-experienced (58%) subjects with HCV genotype 3 infection. The overall SVR12 was 84% and was higher among treatment-naive than treatment-experienced patients (93% versus 77%, respectively). These results suggest higher response rates can be achieved with a 24-week duration of sofosbuvir plus RBV than those reported for the 12- or 16-week durations studied in the FISSION (Lawitz, 2013b) (12 weeks, SVR12: 63%), POSITRON, (Jacobson, 2013c) (12 weeks, SVR 12: 61%) and FUSION (12 weeks, SVR12: 30%, 16 weeks, SVR12: 62%) trials. The primary reason for the higher SVR with extended therapy among treatment-naive patients was a reduction in the relapse rate from 40% to 5%. In sub-analysis, response rates were similarly high among those with (n=45) and without (n=100) cirrhosis (92% and 93%, respectively).
The combination of sofosbuvir plus PEG/RBV has been evaluated in patients with genotype 3 infection. In 2 phase 2 clinical trials, PROTON and ELECTRON, 38 of 39 (97%) treatment-naive patients with genotype 3 infection achieved SVR with sofosbuvir plus PEG (4 to 12 weeks of therapy)/RBV. (Gane, 2013b) For many patients with genotype 3, the adverse effects and increased monitoring requirements of PEG make this less acceptable than the recommended regimen of sofosbuvir plus weight-based RBV.
Although the combination of PEG/RBV is an FDA-approved regimen for HCV genotype 3, its less acceptable adverse effect profile, requirement for more intensive monitoring, and overall lower efficacy make it less desirable than the recommended regimen.
Because of their limited in vitro and in vivo activity against genotype 3, boceprevir, telaprevir, and simeprevir should not be used as therapy for patients with HCV genotype 3 infection.
IV. Genotype 4
Few data are available to help guide decision-making in patients infected with HCV genotype 4. Nonetheless, for those patients for whom immediate treatment is required, the following recommendations have been drawn from available data.
Recommended regimen for treatment-naive patients with HCV genotype 4 who are eligible to receive IFN.
Daily sofosbuvir (400 mg) and weight-based RBV (1000 mg [<75 kg] to 1200 mg [>75 kg]) plus weekly PEG for 12 weeks is recommended for IFN-eligible persons with HCV genotype 4 infection.
Rating: Class IIa, Level B
In the Phase 3 NEUTRINO trial, (Lawitz, 2013b) 28 treatment-naive patients with HCV genotype 4 infection were treated with sofosbuvir (400 mg daily) plus PEG (2a) (180 µg weekly) and weight-based RBV (1000 mg 1200 mg once daily) for 12 weeks. Of the 28 patients with genotype 4, 27 (96%) achieved SVR12. The one patient who did not achieve SVR had cirrhosis and relapsed after therapy. The adverse event profile was similar to that seen with PEG/RBV therapy.
Recommended regimen for treatment-naive patients with genotype 4 who are not eligible to receive IFN.
Daily sofosbuvir (400 mg) plus weight-based RBV (1000 mg [<75 kg] to 1200 mg [>75 kg]) for 24 weeks is recommended for IFN-ineligible IFN ineligible is defined as one or more of the below:
• Intolerance to IFN
• Autoimmune hepatitis and other autoimmune disorders
• Hypersensitivity to PEG or any of its components
• Decompensated hepatic disease
• History of depression, or clinical features consistent with depression
• A baseline neutrophil count below 1500/μL, a baseline platelet count below 90,000/μL or baseline hemoglobin below 10 g/dL
• A history of preexisting cardiac disease patients with HCV genotype 4 infection.
Rating: Class IIb, Level B
In a small study of Egyptian patients in the United States treated with sofosbuvir plus weight-based RBV (1000 mg to 1200 mg), SVR12 was achieved in 11 of 14 (79%) treatment-naive patients treated for 12 weeks; SVR24 was achieved in 100% of the 14 treatment-naive patients treated for 24 weeks. (Ruane, 2013)
Alternative regimens for treatment-naive patients with HCV genotype 4 who are eligible to receive IFN.
Daily simeprevir (150 mg) for 12 weeks and weight-based RBV (1000 mg [<75 kg] to 1200 mg [>75 kg]) plus weekly PEG for 24 to 48 weeks is an alternative regimen for IFN-eligible persons with HCV genotype 4 infection.
Rating: Class IIb, Level B
A Phase 3 trial in patients with HCV genotype 4 is currently under way. This trial compares PEG and weight-based RBV (1000 mg to 1200 mg) for 48 weeks with a 12-week regimen of simeprevir 150 mg once daily plus PEG and weight-based RBV (1000 mg to 1200 mg) followed by an additional 12 or 36 weeks of PEG/RBV alone. (Moreno, 2013) In another study, the RESTORE trial, an RGT approach is used in place of the simeprevir arm. Patients who have HCV RNA below 25 IU/mL at week 4 and undetectable HCV RNA by week 12 continue PEG/RBV for an additional 12 weeks, and those who do not achieve this response continue PEG/RBV for an additional 36 weeks (total 48 weeks of therapy). The study has enrolled 107 patients, of whom 35 are treatment-naive, including 2 with cirrhosis. To date, 10 of 11 patients (91%) who met criteria for shortened therapy have achieved SVR4, and 3 of 3 have achieved SVR12. To date, therapy has failed in 4 patients: 3 had detectable virus at the end of treatment and 1 experienced virologic relapse. Anemia was reported in 8.4% and hyperbilirubinemia in 1.9% of all study participants (n=107) (including treatment-experienced patients). Four serious adverse events were attributed to simeprevir. No episodes of rash were reported. (Moreno, 2013)
The following regimens are NOT recommended for treatment-naive patients with HCV genotype 4.
- PEG/RBV for 48 weeksRating: Class IIb, Level A
- Monotherapy with PEG, RBV, or a DAARating: Class III, Level A
- Telaprevir- or boceprevir-based regimensRating: Class III, Level A
PEG/RBV for 48 weeks was the previously recommended regimen for patients with HCV genotype 4. The addition of sofosbuvir (400 mg daily) to PEG/RBV increases response rates and markedly shortens therapy with no apparent additional adverse effects. The addition of simeprevir to PEG/RBV increases response rates with a minimal increase in adverse events and can shorten therapy to 24 weeks.
Because of their limited in vitro and in vivo activity against genotype 4, boceprevir or telaprevir should not be used as therapy for patients with HCV genotype 4 infection.
V. Genotype 5 or 6
Few data are available to help guide decision-making in patients infected with HCV genotype 5 or 6. Nonetheless, for those patients for whom immediate treatment is required, the following recommendations have been drawn from available data. No data are available to support the use of a non-PEG containing regimen for patients with HCV genotype 5 or 6 infection.
Recommended regimen for treatment-naive patients with HCV genotype 5 or 6.
Daily sofosbuvir (400 mg) and weight-based RBV (1000 mg [<75 kg] to 1200 mg [>75 kg]) plus weekly PEG for 12 weeks is recommended for IFN-eligible persons with HCV genotype 5 or 6 infection.
Rating: Class IIa, Level B
In the Phase 3 NEUTRINO trial (Lawitz, 2013b), treatment-naive patients with genotypes 1 (n=291), 4 (n=28), 5 (n=1), and 6 (n=6) were treated with sofosbuvir (400 mg daily) plus PEG (2a) (180 µg per week) and weight-based RBV (1000 mg 1200 mg once daily) for 12 weeks. All 6 patients with HCV genotype 6 and the 1 patient with genotype 5 achieved SVR12. The adverse event profile in these patients and in the larger study population was similar to that seen with PEG/RBV therapy.
Alternative regimens for treatment-naive patients with HCV genotype 5 or 6.
Daily weight-based RBV (1000 mg [<75 kg] to 1200 mg [>75 kg]) plus weekly PEG for 48 weeks is an acceptable regimen for persons infected with HCV genotype 5 or 6.
Rating: Class IIb, Level A
PEG/RBV for 48 weeks was the previously recommended regimen for patients infected with HCV genotype 5 or 6. Sofosbuvir has activity against genotypes 5 and 6, and when combined with PEG/RBV for 12 weeks led to SVR in the 6 patients in whom it was studied. (Lawitz, 2013b) The addition of sofosbuvir (400 mg daily) to PEG/RBV shortens duration of therapy with no apparent additional adverse effects and likely substantially increases response rates.
The following regimens are NOT recommended for treatment-naive patients with genotype 5 or 6 HCV.
- Monotherapy with PEG, RBV, or a DAARating: Class III, Level A
- Telaprevir- or boceprevir-based regimensRating: Class III, Level A
Because of their limited activity in vitro and in vivo against genotypes 5 and 6, boceprevir or telaprevir should not be used as therapy for patients with genotype 5 or 6 HCV infection.
https://www.hcvguidelines.org/full-report/initial-treatment-hcv-infection-patients-starting-treatment 참고