EASL Recommendations on Treatment of Hepatitis C 2015
https://www.journal-of-hepatology.eu/article/S0168-8278(15)00208-1/fulltext
치료 요약 표 (대한 간학회 KASL가이드라인과 미국간학회 AASLD 가이드라인과 약간 차이가 있습니다.)
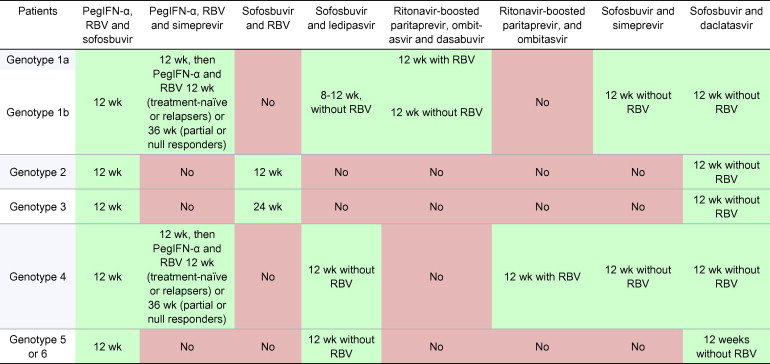
Table 5
Treatment recommendations for HCV-monoinfected or HCV/HIV coinfected patients with chronic hepatitis C without cirrhosis, including treatment-naïve patients and patients who failed on a treatment based on PegIFN-α and ribavirin (RBV).
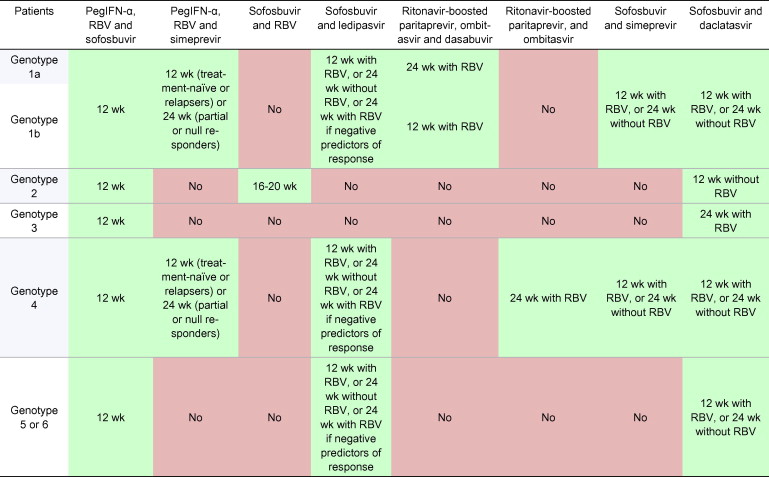
Table 6
Treatment recommendations for HCV-monoinfected or HCV/HIV coinfected patients with chronic hepatitis C with compensated (Child-Pugh A) cirrhosis, including treatment-naïve patients and patients who failed on a treatment based on PegIFN-α and ribavirin (RBV).
Treatment of HCV genotype 1 infection
Six treatment options are available in 2015 for patients infected with HCV genotype 1, including two IFN-containing regimens and four IFN-free regimens. The combination of sofosbuvir and ribavirin should not be used in patients infected with HCV genotype 1. In settings where none of the proposed options is available, the double combination of PegIFN-α and ribavirin, or the triple combination of PegIFN-α, ribavirin and either telaprevir or boceprevir, remain acceptable for selected patients likely to respond to these regimens until new DAAs become available and affordable; see prior EASL Clinical Practice Guidelines
Genotype 1, IFN-containing Option 1 Comments:
This combination has been evaluated in the NEUTRINO Phase III trial in treatment-naïve patients [25]. The overall SVR rate was 89% (259/291), 92% (207/225) for subtype 1a and 82% (54/66) for subtype 1b. Patients with cirrhosis had a lower SVR rate than non-cirrhotic patients (80% vs. 92%, respectively). Patients who failed on this regimen did not select HCV variants resistant to sofosbuvir. No Phase II data with this regimen has been presented in patients who failed prior PegIFN-α and ribavirin treatment. However, based on SVR rates in historical studies and the NEUTRINO trial, the US Food and Drug Administration predicted that 78% of patients who failed prior PegIFN-α and ribavirin treatment would achieve an SVR with the triple combination of PegIFN-α, ribavirin and sofosbuvir (although different models yielded slightly different predictions) [26]. Similarly, there is no data with this regimen in patients who failed prior PegIFN-α, ribavirin and either telaprevir or boceprevir treatment. The SVR12 rate with the triple combination of PegIFN-α, ribavirin and sofosbuvir was 74% in patients who failed to achieve an SVR after receiving PegIFN-α, ribavirin and an investigational protease inhibitor alone or in combination with a non-nucleoside inhibitor of the HCV RNA-dependent RNA polymerase or ledipasvir [27]. There is no data with this combination in HIV-coinfected patients, and relatively small numbers of patients with cirrhosis were included. Whether longer treatment duration would be needed in the most difficult-to-cure population is unknown. Preliminary results from two large-scale US real-life studies have been presented. In HCV TARGET 2.0 [13], the overall SVR4 rate with the triple combination of PegIFN-α, ribavirin and sofosbuvir was 85% (140/164; 55% were treatment-naïve and 45% treatment-experienced patients). SVR4 was achieved in 90% (114/127) of non-cirrhotic patients but 70% (26/37) of cirrhotic patients. In the TRIO real-life study, which included 58% of treatment-naïve and 42% of treatment-experienced patients, SVR12 was achieved in 81% (112/138) of treatment-naïve non-cirrhotic patients and 81% (25/31) of treatment-naïve cirrhotic patients, and in 77% (30/39) of treatment-experienced patients without cirrhosis and 62% (53/85) of treatment-experienced patients with cirrhosis (intent-to-treat) receiving PegIFN-α, ribavirin and sofosbuvir
Genotype 1, IFN-containing Option 2 Comments:
This combination has been evaluated in the QUEST-1 and QUEST-2 Phase III clinical trials in treatment-naïve patients [[29], [30]]. The overall SVR rates were 80% (210/264) and 81% (209/257), respectively. In a pooled analysis of both trials, patients infected with subtype 1b achieved an SVR in 85% of cases (228/267). Patients infected with subtype 1a achieved an SVR in 84% of cases (138/165) when no Q80K substitution was detectable in the NS3 protease sequence at baseline. The SVR was only 58% (49/84) when a Q80K substitution was detectable at baseline by population sequencing. SVR was achieved with this regimen in 84% (317/378) of patients with an F0-F2 METAVIR score, 73% (60/82) of patients with F3, and 60% (29/48) of patients with F4 (cirrhosis). However, for patients who received 24 weeks of treatment, the SVR rate was lower in those with detectable than in those with undetectable HCV RNA at treatment week 4 (69% vs. 93%, respectively) [[29], [30]. In treatment-naïve, HIV-coinfected patients receiving this treatment regimen, SVR was achieved in 79% of patients (42/53) [31]. In monoinfected patients who previously relapsed to IFN-α/ribavirin-based therapy, SVR24 was achieved in 86% (128/149) of subtype 1b patients and in 70% (78/111) of subtype 1a patients. Among patients infected with genotype 1a, SVR24 was achieved in 78% of those without and 47% of those with a detectable Q80K substitution at baseline [32]. The SVR rate in HIV-coinfected relapsers was 87% (13/15) in another study [31].
In the ATTAIN Phase III study, SVR12 was achieved in 70% (101/145) of prior partial responders and 44% (102/234) of null responders to IFN-α/ribavirin-based therapy treated with the triple combination of PegIFN-α, ribavirin and simeprevir, vs. 68% (100/146) and 46% (110/238) of the same groups who received telaprevir, respectively [33]. In HIV-coinfected patients, 70% (7/10) of partial responders and 54% (15/28) of null responders achieved an SVR24 in another study
IFN-free options Genotype 1, IFN-free Option 1
Comments: This recommendation is based on the results of the three Phase III trials ION-1, ION-2 and ION-3 [[34], [35], [36]]. In ION-1, treatment-naïve patients, including 16% with compensated cirrhosis, achieved SVR12 in 99% (211/214) and 97% (211/217) of cases after 12 weeks of the fixed-dose combination of sofosbuvir and ledipasvir without or with ribavirin, respectively. The SVR12 rates were 98% (212/217) and 99% (215/217) after 24 weeks of the same combination without or with ribavirin, respectively [34]. In ION-3 in treatment-naïve patients without cirrhosis (F3 in only 13% of patients who underwent liver biopsy), the SVR12 rates were 94% (202/215) without ribavirin for 8 weeks, 93% (201/216) with ribavirin for 8 weeks and 95% (205/216) without ribavirin for 12 weeks. The absolute number of post-treatment relapses was, however, higher in the 8-weeks arms: 11/215, 9/216 and 3/216, respectively. Post hoc analysis indicated that only patients with an HCV RNA level <6 million (6.8 Log) IU/ml at baseline could be treated for 8 weeks [36]. However, HCV RNA level determination can be inaccurate within this range of values with currently available HCV RNA assays and real-life confirmation is needed to determine that 8 weeks of treatment with this combination is sufficient. Interestingly, the relapse rates were 1% (1/84) and 1% (1/96) in female patients treated for 8 weeks with sofosbuvir and ledipasvir without and with ribavirin, respectively, and 8% (10/129) and 7% (8/114) in males, respectively, in the ION-3 study [36]. In another Phase II study, the combination of sofosbuvir and ledipasvir was given for 12 weeks without ribavirin to patients with HCV genotype 1 infection coinfected with HIV, including 13 not treated for their HIV infection and 37 receiving antiretroviral therapy. All but one patient (98%) achieved an SVR12 [37].
In ION-2, in treatment-experienced patients (prior PegIFN-α and ribavirin or PegIFN-α, ribavirin and either telaprevir or boceprevir), including 20% with cirrhosis, the SVR12 rates were 94% (102/109) and 96% (107/111) without or with ribavirin, respectively. After 24 weeks of therapy, SVR rates were 99% (108/109) and 99% (110/111), respectively [37].
An integrated analysis of 513 genotype 1 patients with compensated cirrhosis treated with the fixed-dose combination of sofosbuvir and ledipasvir, with or without ribavirin, in different Phase II and III studies showed overall SVR12 rates of 95% (305/322) after 12 weeks and 98% (188/191) after 24 weeks of therapy [38]. Neither treatment duration nor ribavirin had an impact on SVR12 in treatment-naïve patients (SVR12 rates between 96% and 100%). In contrast, in treatment-experienced patients, the SVR12 rates were 90% after 12 weeks without ribavirin, 96% after 12 weeks with ribavirin, 98% after 24 weeks without ribavirin, and 100% after 24 weeks with ribavirin. A platelet count <75 × 103/μl was associated with a lower rate of SVR among treatment-experienced patients (based on 28 patients) [38]. In the SIRIUS study, 12 weeks of the fixed-dose combination of sofosbuvir and ledipasvir with ribavirin or 24 weeks of the same combination without ribavirin in patients with compensated cirrhosis who failed to achieve an SVR after treatment with PegIFN-α, ribavirin and either telaprevir or boceprevir yielded SVR12 rates of 96% (74/77) and 97% (75/77), respectively [39].
Genotype 1, IFN-free Option 2 Comments:
This recommendation is based on the results of seven Phase III trials. In SAPPHIRE-I in treatment-naïve patients without cirrhosis treated with this combination together with ribavirin for 12 weeks, the SVR12 rates were 95% (307/322) in subtype 1a and 98% (148/151) in subtype 1b patients [40]. In PEARL-IV, the SVR12 rates were 90% (185/205) and 97% (97/100) without and with ribavirin, respectively, in treatment-naïve non-cirrhotic patients infected with subtype 1a. In PEARL-III, the SVR12 rates were 99% (207/209) and 99% (209/210) without and with ribavirin, respectively, in treatment-naïve non-cirrhotic patients infected with subtype 1b [41]. In the TURQUOISE-I study in treatment-naïve, non-cirrhotic patients coinfected with HIV-1 and stable on antiretroviral treatment containing atazanavir or raltegravir, the SVR12 rates were 93% (29/31) and 91% (29/32) after 12 or 24 weeks of treatment, respectively; SVR12 was achieved in 91% (51/56) of subtype 1a and 100% (7/7) of subtype 1b patients [42].
In non-cirrhotic treatment-experienced patients (PegIFN-α and ribavirin failures) treated with this combination with ribavirin for 12 weeks in SAPPHIRE-II, the SVR12 rates were 96% (166/173) in subtype 1a and 97% (119/123) in subtype 1b patients. Overall, the SVR12 rates were 95% (82/86) in prior relapsers, 100% (65/65) in prior partial responders and 95% (139/146) in prior null responders [43]. SVR12 was achieved in 100% (91/91) of cases without ribavirin and 97% (85/88) with ribavirin in patients infected with subtype 1b receiving this combination in the PEARL-II trial [44].
In treatment-naïve and treatment-experienced patients with compensated cirrhosis, the rates of SVR were 92% (191/208) after 12 weeks and 96% (165/172) after 24 weeks of the combination of ritonavir-boosted paritaprevir, ombitasvir and dasabuvir plus ribavirin in the TURQUOISE-II trial. SVR12 was achieved in 92% (239/261) of genotype 1a and 99% (118/119) of genotype 1b patients [45]. In patients with α-fetoprotein level <20 ng/ml, platelet count ⩾90 × 109/L and albumin level ⩾35 g/L prior to treatment, the relapse rates were 1% (1/87) and 0% (0/68) after 12 or 24 weeks of treatment, respectively; in patients with α-fetoprotein level ⩾20 ng/ml and/or platelet count <90 × 109/L and/or albumin level <35 g/L prior to treatment, they were 21% (10/48) and 2% (1/45) after 12 or 24 weeks of treatment, respectively [45].
Genotype 1, IFN-free Option 3 Comments:
This recommendation is based on results from the COSMOS Phase IIb trial [11]. In the first cohort, 80 prior null responders to PegIFN-α and ribavirin therapy with a METAVIR score F0 to F2 were treated 12 or 24 weeks, with or without ribavirin. The SVR12 rates were 93% (13/14) and 96% (26/27) for 12 weeks of therapy without and with ribavirin, respectively, and 93% (14/15) and 79% (19/24) for 24 weeks of therapy without and with ribavirin, respectively. In the second cohort, 87 treatment-naïve patients and prior null responders with a METAVIR score of F3–F4 were treated 12 or 24 weeks, without or with ribavirin. The SVR12 rates were 93% (13/14) and 93% (25/27) for 12 weeks of therapy without and with ribavirin, respectively, and 100% (16/16) and 93% (28/30) for 24 weeks of therapy without and with ribavirin, respectively. All virological failures were due to post-treatment relapses [11].
Preliminary results from two large-scale US real-life studies with sofosbuvir and simeprevir indicate that this combination is well tolerated and yields high SVR rates, which are however lower than those reported in the COSMOS trial, in particular in patients with advanced stages of liver disease [[13], [28]]. These studies are not conclusive as to the value of adding ribavirin to the sofosbuvir-simeprevir combination (ribavirin addition was at the prescriber’s discretion and may have been influenced by various pretreatment parameters). In HCV TARGET 2.0 [13], the overall SVR4 rate was 89% (269/303). SVR4 was achieved in 92% (113/123) of non-cirrhotic patients, 87% (156/180) of cirrhotic patients, and 75% (61/81) of cirrhotic patients with prior decompensation. SVR4 was more frequent in subtype 1b than 1a patients: 95% (88/93) and 89% (47/53), respectively. SVR4 was achieved in 81% (44/54) of patients who failed on a prior treatment with PegIFN-α, ribavirin and either telaprevir or boceprevir, including in 85% (17/20) of non-cirrhotic patients and 79% (27/34) of cirrhotic patients. Preliminary data from the TRIO real-life study showed SVR12 in 88% (68/88) of treatment-naïve non-cirrhotic and 75% (41/55) of treatment-naïve cirrhotic patients; SVR rates were 87% (64/74) and 76% (53/70) in treatment-experienced non-cirrhotic and cirrhotic patients, respectively (intent-to-treat) [28].
Genotype 1, IFN-free Option 4 Comments:
Phase IIb results have been published with this combination in patients without cirrhosis [14]. With 24 weeks of therapy, the SVR rates were 100% (14/14 and 15/15, without and with ribavirin, respectively) in treatment-naïve patients, and 100% (21/21) and 95% (19/21) without and with ribavirin, respectively, in patients who did not respond to the combination of PegIFN-α, ribavirin, and either telaprevir or boceprevir. With 12 weeks of therapy, SVR was achieved in 98% (40/41) of treatment-naïve patients without ribavirin (the remaining patient was lost to follow-up) [14]. Large-scale real-life data from European early access programmes will be presented in 2015
Genotype 2, Option 1 Comments:
Results from four Phase III trials have been published. In the FISSION trial in treatment-naïve patients treated 12 weeks [25], the SVR rate was 95% (69/73). The response rate was better in patients without cirrhosis (97% vs. 83% in patients without and with cirrhosis, respectively). The POSITRON trial included patients considered ineligible or intolerant to IFN, who were treated for 12 weeks with sofosbuvir and ribavirin [46]. SVR was achieved in 93% (101/109) of cases. When comparing 12 and 16 weeks of therapy in the FUSION trial [46], SVR was achieved in 82% (32/39) and 89% (31/35) of cases, respectively, 60% (6/10) and 78% (7/9) in patients with cirrhosis, respectively. This indicates that patients with cirrhosis may benefit from longer than 12 weeks of therapy. In the VALENCE trial [47], the SVR rates after 12 weeks of treatment were 97% (29/30) in treatment-naïve non-cirrhotic individuals, 100% (2/2) in treatment-naïve cirrhotic patients, 91% (30/33) in treatment-experienced non-cirrhotic patients, and 88% (7/8) in treatment-experienced cirrhotic patients. In another study, 1 of 2 patients who relapsed after treatment with sofosbuvir and ribavirin retreated 24 weeks with sofosbuvir and ribavirin achieved an SVR [48]. The combination of sofosbuvir and ribavirin was well tolerated in all these studies. No virological breakthroughs were observed in treatment-adherent patients, and relapses were not associated with the selection of resistant HCV variants.
Genotype 2, Option 2 Comments:
In the LONESTAR-2 Phase IIb study [49], a single centre study in which 23 treatment-experienced patients infected with HCV genotype 2, including 14 with cirrhosis, received 12 weeks of PegIFN-α, ribavirin and sofosbuvir, the SVR rate was 96%. In another study, 4/4 patients who relapsed after treatment with sofosbuvir and ribavirin retreated 12 weeks with the triple combination of PegIFN-α, ribavirin and sofosbuvir achieved an SVR [48].
Genotype 2, Option 3 Comments:
Daclatasvir is active against genotype 2 in vitro. In a Phase II trial, 92% (24/26) of patients infected with genotype 2 achieved an SVR12 after 24 weeks of sofosbuvir and daclatasvir. Based on data with other, more difficult-to-cure HCV genotypes, 12 weeks is probably sufficient for this regimen that should be reserved for patients who failed with other options. Treatment of HCV genotype 3 infection Three treatment options are available for patients infected with HCV genotype 3. The combination of sofosbuvir and ribavirin is suboptimal, in particular in patients with cirrhosis who have previously failed IFN and ribavirin. Based on data with other genotypes and results in a small group of genotype 3-infected patients, the triple combination of PegIFN-α, ribavirin and sofosbuvir appears to be valuable. The IFN-free combination of sofosbuvir and daclatasvir, with or without ribavirin, is another attractive option for patients infected with HCV genotype 3.
Ledipasvir is considerably less potent against genotype 3 than daclatasvir in vitro; in clinical trials with ledipasvir, the respective roles of ledipasvir and ribavirin in combination with sofosbuvir cannot be determined in the absence of control arms with sofosbuvir and ribavirin alone. Thus, although this combination has been used, pending further studies in larger populations including appropriate control arms, the combination of sofosbuvir plus ledipasvir is not recommended in patients infected with HCV genotype 3.
In settings where none of these options is available, the combination of PegIFN-α and ribavirin remains acceptable, according to previous EASL Clinical Practice Guidelines [5].
Genotype 3, Option 1 Comments:
This combination has been evaluated in 10 treatment-naïve non-cirrhotic patients infected with genotype 3. Nine of them achieved an SVR, whereas the remaining one was lost to follow-up [50]. In addition, data with this combination in patients infected with HCV genotype 3 are available from the LONESTAR-2 Phase IIb trial in treatment-experienced individuals [49], who achieved an SVR in 83% (20/24) of cases, including 10/12 patients with cirrhosis. However, the pangenotypic activity of sofosbuvir together with high SVR rates with other genotypes (89% (259/291) overall for genotypes 1 and 4 to 6) indicate that this combination can be safely used in patients infected with HCV genotype 3. In another study, patients infected with genotype 3 who relapsed after treatment with sofosbuvir and ribavirin retreated with the triple combination of PegIFN-α, ribavirin and sofosbuvir for 12 weeks achieved an SVR in 91% (20/22) of cases [48].
Genotype 3, Option 2 Comments:
Results from four Phase III trials have been published. In the FISSION trial in treatment-naïve patients treated 12 weeks [25], the SVR rate was 56% (102/183). The response rate was better in patients without cirrhosis (61% and 34% in patients without and with cirrhosis, respectively). The POSITRON trial included patients ineligible or intolerant to IFN-based therapy who were treated for 12 weeks with sofosbuvir and ribavirin [46]; SVR was achieved in 61% (60/98) of cases. When comparing 12 and 16 weeks of therapy in the FUSION trial [46], SVR was achieved in 30% (19/64) and 62% (39/63) of cases, respectively, 19% (5/26) and 61% (14/23) in patients with cirrhosis, respectively. In the VALENCE trial [51], the SVR rates after 24 weeks of treatment were 94% (86/92) in treatment-naïve non-cirrhotic individuals, 92% (12/13) in treatment-naïve cirrhotics, 87% (87/100) in treatment-experienced non-cirrhotic patients, and 60% (27/45) in treatment-experienced cirrhotic patients. These results indicate that 24 weeks is the appropriate duration for this regimen in patients infected with HCV genotype 3, and that this regimen is suboptimal in treatment-experienced patients with cirrhosis. In another study, patients infected with genotype 3 who relapsed after treatment with sofosbuvir and ribavirin and were retreated 24 weeks with sofosbuvir and ribavirin achieved an SVR in only 63% (24/38) of cases, indicating that this regimen is suboptimal in such patients [48].
The combination of sofosbuvir and ribavirin was well tolerated and very few patients stopped therapy. No virological breakthroughs were observed in treatment-adherent patients, and relapses were not associated with the selection of resistant HCV variants. Genotype 3, Option 3 Comments: In a Phase IIb trial with this combination for 24 weeks [14], the SVR rate was 89% (16/18) in treatment-naïve non-cirrhotic patients infected with HCV genotype 3. In the ALLY-3 Phase III trial, patients were treated for 12 weeks with the combination of sofosbuvir and daclatasvir, without ribavirin. The SVR12 rates were 97% (73/75) and 58% (11/19) in treatment-naïve non-cirrhotic and cirrhotic patients, respectively; they were 94% (32/34) and 69% (9/13) in treatment-experienced non-cirrhotic and cirrhotic patients, respectively [52]. This regimen was well tolerated, with rare adverse events, none of which led to treatment discontinuation. The impact of pre-existing substitutions in the NS5A protein sequence known to confer resistance to daclatasvir at baseline on the response is unknown with this genotype